Abstract
Introduction: Complete excision of early stage pancreatic neuroendocrine tumors (PNETs) is curative. However, small PNETs may be difficult to localize intraoperatively, leading to larger pancreatic resections than required. Prior reports suggest that preoperative endoscopic ultrasound guided placement of markers (fiducials) (EUS-F) facilitates tumor identification during open surgery. We describe our experience with this approach during minimally invasive surgery to preserve pancreatic parenchyma.
Methods: This is a retrospective case series of 3 patients since 2010 who underwent EUS-F followed by laparoscopic or robotic pancreatic resection of PNETs ≤2.5 cm. All patients underwent preoperative EUS-F by our gastroenterology service. We report the visibility of fiducials at surgery by ultrasound and/or fluoroscopy as well as their impact on the extent of surgery.
Results: Patients received 1 to 3 markers without complication. Final pathology revealed complete excision of a 25 mm nonfunctional uncinate tumor, 22 mm tail insulinoma, and 2.5 mm nonfunctional tail tumor. The uncinate tumor was identified using intraoperative ultrasound and enucleated. The two tail tumors were identified using fluoroscopy after failed attempts with ultrasound; these two patients underwent limited distal pancreatectomies. There was one self-limited pancreatic fistula.
Conclusions: Endoscopically placed fiducials facilitate reliable intra-operative identification of small PNETs allowing for minimally invasive parenchymal preserving surgery, such as enucleation or limited distal pancreatectomy. Fluoroscopy was the most reliable method of identifying the fiducials.
Key words
enucleation, fiducial, pancreatic neuroendocrine tumor
Introduction
Pancreatic neuroendocrine tumors (PNETs) are rare, affecting 1 per 500,000 people, and may cause an increased production of hormones such as insulin, gastrin, glucagon, vasoactive intestinal peptide, and somatostatin [1]. PNETs are becoming more frequently diagnosed, with a 710% increase over the last 22 years, mostly due to the widespread use of high resolution imaging [2,3].
PNETs are staged according to size, lymph node involvement, and presence of distant metastases [4]. PNETs are graded histologically according to their degree of differentiation [4]. Early stage and low grade PNETs have the most favorable prognosis- 100% 5 year survival rate for stage I compared to 55% for stage IV.5 Complete surgical resection is curative for these tumors [6,7]. For all stages and grades of PNETs, survival is better in those who undergo surgical resection [8].
Small PNETS (<15mm) have very little risk of recurrence, lymph node involvement, or distant metastases [9-12]. These tumors should be resected with a parenchymal-preserving technique (enucleation) since they would not benefit from more extensive resections such as pancreaticoduodenectomy or distal pancreatectomy. While enucleation does results in a higher fistula rate (21%) requiring prolonged drainage compared to pancreaticoduodenectomy, it also results in fistulas of less severity (International Study Group Pancreatic Fistula Type A), less operative blood loss, less operative time, shorter length of intensive care unit stay, and shorter length of overall hospital stay [13-15]. Pancreaticoduodenectomy also results in new onset exocrine (1.5%) and endocrine (4%) insufficiencies that are not associated with enucleation [16-19]. Enucleation has been accepted as a standard surgical approach for these tumors since 1990 [20]. Most PNETs (56%) are located in the pancreatic head/uncinate region [21]. Tumors of this area that are oncologically amenable to enucleation should proceed as such in order to avoid the complications associated with pancreaticoduodenectomy [15,19].
Minimally invasive pancreatic surgery and pancreatic parenchymal-sparing surgery for PNETs has become more popular and have proven to be feasible, safe, and reduce length of hospital stay, without increasing morbidity or compromising survival compared to its open counterpart [22-30]. Small, deep PNETs in the pancreatic uncinate may be difficult to localize intra-operatively and may explain why PNETs of the pancreatic head are less often resected and technically more challenging to enucleate laparoscopically [31].
Preoperative localization of pancreatic neuroendocrine tumors with traditional axial imaging fails in 40-60% of patients. Endoscopic ultrasound (EUS) is highly sensitive (93%) in the detection of these PNETs [21]. Intra-operative localization with laparoscopic ultrasound has been shown to reduce the need for conversion to an open procedure to identify and guide resection of these tumors [30,32-35]. X-ray and echo-opaque markers (fiducials) are added to guide stereotactic body radiation therapy and lessening collateral damage [36]. These markers have been delivered via EUS for mediastinal and abdominal tumors and more recently applied safely, effectively, and specifically to the pancreas (2% complications, 7% migration, and 90% successful placement). These markers have aided open enucleation of pancreatic uncinate PNETs [37-40].
The aim of this report is to address the intra-operative localization facilitated by endoscopic ultrasound placed fiducials and the operative technique of laparoscopic enucleation of the PNET of the uncinate.
Case report
A 56 year old gentleman with a history of benign prostatic hypertrophy presented with hematuria. He was incidentally diagnosed with a 10 mm tumor in the pancreatic uncinate on computed tomography (Figure 1) without any symptoms or other concerning radiographic findings. He underwent further imaging with endoscopic ultrasound, which documented a well circumscribed oval-shaped 10 x 12 mm tumor without any suspicious appearing regional lymph nodes. A trans-duodenal EUS-guided biopsy of this tumor revealed a neuroendocrine tumor with cells testing positive for CD56, synaptophysin, chromogranin, and pan cytokeratin. Because of its low stage, laparoscopic enucleation was planned. Due to its small size and remote location in the uncinate, 3 x-ray and echo-opaque markers (Visicoil fiducials, Core Oncology, Santa Barbara, CA, USA) were placed in and around the tumor the day prior to surgery via a 22-gauge Cook Echo Tip® needle under EUS-guidance (Figure 2). No complications were associated with the placement of the fiducials, and no evidence of pancreatitis was shown either clinically or on surgical pathology. Final pathology confirmed an early stage (1B, 2.5 x 2.0 x 2.0 cm) and low grade (moderate differentiation) neuroendocrine tumor confined to pancreas with 1 mitosis per 2 square mm, without tumor necrosis or lymphatic-vascular or perineural invasion and image cytometry of Ki-67 labeling index of proliferation of 9%.
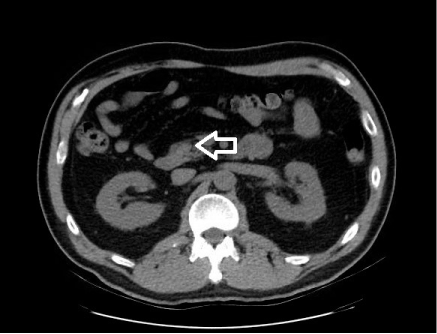
Figure 1. Computed tomography demonstrating uncinate tumor (white arrow)
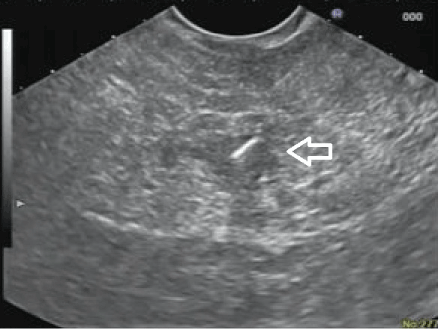
Figure 2. Endoscopic ultrasound guided placement of fiducial (white arrow)
Procedure
Access: Left upper abdominal quadrant Veress technique. Five mm port placed at the site of the Veress entry and 5 mm 30 degree angled laparoscope used to inspect the abdomen.
Ports: The patient was placed in steep reverse Trendelenburg position with left side slightly higher. Two 5 mm ports were placed in the right upper abdominal quadrant, and one 5 mm port was placed in the midline below the xiphoid process. A 10 mm port was placed in the midline above the umbilicus.
Exposure: A 5 mm flexible liver retractor was placed through the infra-xiphoid port which maintained the gallbladder and right lobe of the liver retracted cephalad. The transverse colon was reflected caudad and the duodenum was carefully reflected laterally. The lower border of the pancreas was identified and the entry point of the superior mesenteric vein (SMV) was seen at the level of the pancreatic neck and the uncinate process inferior-laterally.
Localization: The laparoscopic probe for the ultrasound was used to localize this small deep tumor in the uncinate. The fiducials were clearly visible even before the tumor was appreciated in its entirety.
Enucleation: Using the “L” hook on the Bovie® approximately 3 mm of superficial pancreatic tissue was dissected through to reach the tumor. Repeat visualization of the fiducial guided the dissection. Vascular and ductal structures were avoided with the aid of intra-operative ultrasound. The tumor was well circumscribed and was “shelled out” in its entirety following its natural cleavage plane. The specimen was extracted in an Endo Catch™ (Covidien) bag through the 10 mm port.
Confirmation: We confirmed the presence of the fiducials and tumor extra-corporeally with the ultrasound over the specimen. We confirmed the presence of the fiducials by gross pathology (Figure 3) and the complete resection of the neuroendocrine tumor with negative margins on frozen section.
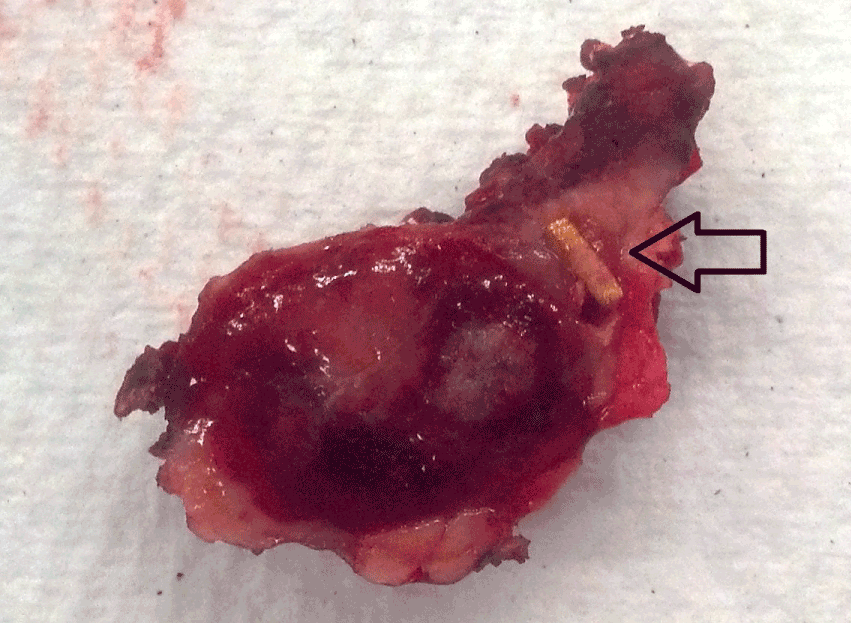
Figure 3. Gross pathology of tumor and fiducial (black arrow)
Closure: The resection area was plugged with a tongue of vascularized omentum which was secured with an absorbable suture. A 19 French BLAKE® silicone drain (Ethicon) was placed in the resection field. The end of the drain was brought out the most lateral 5 mm port. The fascial defect of the 10 mm port was closed. The operative time for the entire procedure including intra-operative pathologic evaluation was approximately 240 minutes with an estimated blood loss of less than 50 ml.
Clinical course
The aforementioned patient progressed without incident until postoperative day 6 when he developed a fever and abdominal pain. A computed tomography identified a 7 cm fluid collection, which was drained and broad spectrum antibiotics were started along with ocreotide. An endoscopic retrograde cholagiopancreatography (ERCP) revealed a fistula for which a stent was placed in the ventral duct. The patient was subsequently discharged home with a low-output fistula (<150cc /day) on postoperative day 11. One month later, drain output decreased to a scant amount. Repeat ERCP revealed resolution of the fistula, and the drain was removed. At one and two year follow up the patient is without sequela of disease or complication, and magnetic resonance imaging without evidence of residual or recurrent tumor.
2021 Copyright OAT. All rights reserv
Patient B. Fifty-four year old female with vague abdominal pain was found to have a 4 mm PNET in the tail on axial imaging. She underwent a laparoscopic distal pancreatectomy. The tumor was not able to be visualized with intraoperative ultrasound but was easily identified with intraoperative fluoroscopy. The precise localization of this tumor allowed for parenchymal preservation via a limited resection of just the portion of the pancreatic tail with splenic preservation where the tumor was located. On final pathology the tumor was only 2.5 mm in maximal dimension. The patient had an uneventful recovery and was discharged on the sixth postoperative day. There were no postoperative indicators of pancreatic insufficiency.
Patient C. Seventy-two year old female with multiple comorbidities (morbid obesity, DM, HTN, CKD, PE) and a 16mm symptomatic insulinoma in the mid-body of the pancreas based on a CT scan. Intraoperative ultrasound and fluoroscopy identified the tumor more distal than previously thought, again allowing for greater parenchymal preservation via limited distal pancreatectomy with splenic preservation. The tumor was 22mm on final pathology. The patient had an uneventful recovery and was discharged on the eighth postoperative day. There were no postoperative indicators of pancreatic insufficiency.
Discussion
This is the first case series which reports the minimally invasive resection of pancreatic neuroendocrine tumors facilitated by preoperative endoscopic ultrasound placement of fiducials. Laparoscopic enucleation of pancreatic tumors has been performed for over 20 years [41,42]. More recently this approach has become quite popular for PNETs [24-30, 43,44]. While there have been reports of laparoscopic resection of the entire uncinate process for relatively larger tumors these are still quite uncommon [45-47]. Two factors continue to limit the widespread application of laparoscopic enucleation of PNETs of the pancreatic head and uncinate. The first factor is difficulty in localizing small and deep tumors intra-operatively. We believe that preoperatively EUS-placed fiducials will facilitate intraoperative localization using ultrasound and/or fluoroscopy (Figure 4) and guide a parenchymal persevering resection based on our growing experience and the report of its use in open enucleation of PNETs of the uncinate [40]. The second factor is the high pancreatic fistula rate. It has been stated with regard to laparoscopic enucleation that “laparoscopy seems to be of no use in right-sided procedures. Pancreatic fistula is still the main cause of long-lasting morbidity”[48]. Factors associated with increased pancreatic fistula development after enucleation include tumor depth (73% in depths >3 mm vs. 30% for superficial tumors) and tumor proximity to the main pancreatic duct (60% ≤2 mm versus 19 % >2 mm) [49,50].
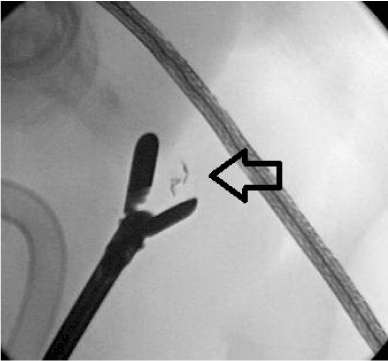
Figure 4. Intraoperative fluoroscopy identifying the fiducial (black arrow)
In the future we hope there will be enough cases to objectively validate this proof-of-concept report. Until then, the clinical benefits and cost effectiveness of this technique are anecdotal.
Conclusions
We demonstrate that the pre-operative placement of EUS-guided fiducials facilitates the successful minimally invasive (laparoscopic and/or robotic) parenchymal preserving (enucleation and/or limited distal pancreatectomy) of a small tumors of the pancreas. High volume pancreatic centers, gastroenterologists performing advanced endoscopic procedures, and pancreatic surgeons with an advanced minimally invasive surgical skill set will benefit most from this specialized approach.
References
- Halfdanarson TR, Rabe KG, Rubin J, Petersen GM (2008) Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol 19: 1727-1733. [Crossref]
- Kuo EJ, Salem RR (2013) Population-level analysis of pancreatic neuroendocrine tumors 2 cm or less in size. Ann Surg Oncol 20: 2815-2821. [Crossref]
- Vagefi PA, Razo O, Deshpande V, McGrath DJ, Lauwers GY, et al. (2007) Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms: the Massachusetts General Hospital experience from 1977 to 2005. Arch Surg 142: 347-354. [Crossref]
- Edge SB, Byrd DR, Compton CC (2010) et al. Exocrine and endocrine pancreas. In: AJCC Cancer Staging Manual. 7th ed. New York. 241-249.
- Pape UF, Jann H, Muller-Nordhorn J, Bockelbrink A, Berndt U, et al. (2008) Prognostic relevance of a novel TNM classification system for upper gastroenteropancreatic neuroendocrine tumors. Cancer 113: 256–265. [Crossref]
- Azimuddin K, Chamberlain RS (2001) The surgical management of pancreatic neuroendocrine tumors. Surg Clin North Am 81: 511-525. [Crossref]
- Kouvaraki MA, Solorzano CC, Shapiro SE, Yao JC, Perrier ND, et al. (2005) Surgical treatment of non-functioning pancreatic islet cell tumors. J Surg Oncol 89: 170-185. [Crossref]
- Hill JS, McPhee JT, McDade TP, Zhou Z, Sullivan ME, et al. (2009) Pancreatic neuroendocrine tumors: the impact of surgical resection on survival. Cancer 115: 741-751. [Crossref]
- Kishi Y, Shimada K, Nara S, Esaki M, Hiraoka N, et al. (2014) Basing treatment strategy for non-functional pancreatic neuroendocrine tumors on tumor size. Ann Surg Oncol 21: 2882-2888. [Crossref]
- Curran T, Pockaj BA, Gray RJ, Halfdanarson TR, Wasif N (2015) Importance of lymph node involvement in pancreatic neuroendocrine tumors: impact on survival and implications for surgical resection. J Gastrointest Surg 19: 152-160. [Crossref]
- Hashim YM, Trinkaus KM, Linehan DC, Strasberg SS, Fields RC, et al. (2014) Regional lymphadenectomy is indicated in the surgical treatment of pancreatic neuroendocrine tumors (PNETs). Ann Surg 259: 197-203. [Crossref]
- Cherenfant J, Stocker SJ, Gage MK, Du H, Thurow TA, et al. (2013) Predicting aggressive behavior in nonfunctioning pancreatic neuroendocrine tumors. Surgery 154: 785-791. [Crossref]
- Bassi C, Dervenis C, Butturini G, Fingerhut A, Yeo C, et al. (2005) Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 138: 8-13. [Crossref]
- Pitt SC, Pitt HA, Baker MS, Christians K, Touzios JG, et al. (2009) Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg 13: 1692-1698. [Crossref]
- Hackert T, Hinz U, Fritz S, Strobel O, Schneider L, et al. (2011) Enucleation in pancreatic surgery: indications, technique, and outcome compared to standard pancreatic resections. Langenbecks Arch Surg 396: 1197-1203. [Crossref]
- Crippa S, Bassi C, Salvia R, Falconi M, Butturini G, et al. (2007) Enucleation of pancreatic neoplasms. Br J Surg 94: 1254-1259. [Crossref]
- Crippa S, Boninsegna L, Partelli S, Falconi M (2010) Parenchyma-sparing resections for pancreatic neoplasms. J Hepatobiliary Pancreat Sci 17: 782-787. [Crossref]
- Crippa S, Zerbi A, Boninsegna L, Capitanio V, Partelli S, et al. (2012) Surgical management of insulinomas: short- and long-term outcomes after enucleations and pancreatic resections. Arch Surg 147: 261-266. [Crossref]
- Falconi M, Zerbi A, Crippa S, Balzano G, Boninsegna L, et al. (2010) Parenchyma-preserving resections for small nonfunctioning pancreatic endocrine tumors. Ann Surg Oncol 17: 1621-1627. [Crossref]
- Norton JA, Shawker TH, Doppman JL, Miller DL, Fraker DL, et al. (1990) Localization and surgical treatment of occult insulinomas. Ann Surg 212: 615-620. [Crossref]
- Anderson MA, Carpenter S, Thompson NW, Nostrant TT, Elta GH, et al. (2000) Endoscopic ultrasound is highly accurate and directs management in patients with neuroendocrine tumors of the pancreas. Am J Gastroenterol 95: 2271-2277. [Crossref]
- DiNorcia J, Lee MK, Reavey PL, Genkinger JM, Lee JA, et al. (2010) One hundred thirty resections for pancreatic neuroendocrine tumor: evaluating the impact of minimally invasive and parenchyma-sparing techniques. J Gastrointest Surg 14: 1536-1546. [Crossref]
- Watzka FM, Laumen C, Fottner C, Weber MM, Schad A, et al. (2013) Resection strategies for neuroendocrine pancreatic neoplasms. Langenbecks Arch Surg 398: 431-440. [Crossref]
- Fernández-Cruz L, Blanco L, Cosa R, Rendón H (2008) Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg 32: 904-917. [Crossref]
- Fernández-Cruz L, Molina V, Vallejos R, Jiménez Chavarria E, López-Boado MA, et al. (2012) Outcome after laparoscopic enucleation for non-functional neuroendocrine pancreatic tumours. HPB (Oxford) 14: 171-176. [Crossref]
- Drymousis P, Raptis DA, Spalding D, Fernandez-Cruz L, Menon D, et al. (2014) Laparoscopic versus open pancreas resection for pancreatic neuroendocrine tumours: a systematic review and meta-analysis. HPB (Oxford) 16: 397-406. [Crossref]
- Su AP, Ke NW, Zhang Y, Liu XB, Hu WM, et al. (2014) Is laparoscopic approach for pancreatic insulinomas safe? Results of a systematic review and meta-analysis. J Surg Res 186: 126-134. [Crossref]
- Hu M, Zhao G, Luo Y, Liu R (2011) Laparoscopic versus open treatment for benign pancreatic insulinomas: an analysis of 89 cases. Surg Endosc 25: 3831-3837. [Crossref]
- Karaliotas C, Sgourakis G (2009) Laparoscopic versus open enucleation for solitary insulinoma in the body and tail of the pancreas. J Gastrointest Surg 13: 1869. [Crossref]
- Roland CL, Lo CY, Miller BS, Holt S, Nwariaku FE (2008) Surgical approach and perioperative complications determine short-term outcomes in patients with insulinoma: results of a bi-institutional study. Ann Surg Oncol 15: 3532-3537. [Crossref]
- Bilimoria KY, Tomlinson JS, Merkow RP, Stewart AK, Ko CY, et al. (2007) Clinicopathologic features and treatment trends of pancreatic neuroendocrine tumors: analysis of 9,821 patients. J Gastrointest Surg 11: 1460-1467. [Crossref]
- Ayav A, Bresler L, Brunaud L, Boissel P; SFCL (Société Française de Chirurgie Laparoscopique); AFCE (Association Francophone de Chirurgie Endocrinienne) (2005) Laparoscopic approach for solitary insulinoma: a multicentre study. Langenbecks Arch Surg 390: 134-140. [Crossref]
- Sa Cunha A, Beau C, Rault A, Catargi B, Collet D, et al. (2007) Laparoscopic versus open approach for solitary insulinoma. Surg Endosc 21: 103-108. [Crossref]
- Spitz JD, Lilly MC, Tetik C, Arregui ME (2000) Ultrasound-guided laparoscopic resection of pancreatic islet cell tumors. Surg Laparosc Endosc Percutan Tech 10: 168-173. [Crossref]
- Lo CY, Lo CM, Fan ST (2000) Role of laparoscopic ultrasonography in intraoperative localization of pancreatic insulinoma. Surg Endosc 14: 1131-1135. [Crossref]
- Jin Z, Chang KJ (2012) Endoscopic ultrasound-guided fiducial markers and brachytherapy. Gastrointest Endosc Clin N Am 22: 325-331. [Crossref]
- Pishvaian AC, Collins B, Gagnon G, Ahlawat S, Haddad NG (2006) EUS-guided fiducial placement for CyberKnife radiotherapy of mediastinal and abdominal malignancies. Gastrointest Endosc 64: 412-417. [Crossref]
- Park WG, Yan BM, Schellenberg D, Kim J, Chang DT, et al. (2010) EUS-guided gold fiducial insertion for image-guided radiation therapy of pancreatic cancer: 50 successful cases without fluoroscopy. Gastrointest Endosc 71: 513-518. [Crossref]
- Sanders MK, Moser AJ, Khalid A, Fasanella KE, Zeh HJ, et al. (2010) EUS-guided fiducial placement for stereotactic body radiotherapy in locally advanced and recurrent pancreatic cancer. Gastrointest Endosc 71: 1178-1184. [Crossref]
- Law JK, Singh VK, Khashab MA, Hruban RH, Canto MI, et al. (2013) Endoscopic ultrasound (EUS)-guided fiducial placement allows localization of small neuroendocrine tumors during parenchymal-sparing pancreatic surgery. Surg Endosc 27: 3921-3926. [Crossref]
- Cuschieri A (1996) Laparoscopic Pancreatic Resections. Semin Laparosc Surg 3: 15-20. [Crossref]
- Gagner M, Pomp A, Herrera MF (1996) Early experience with laparoscopic resections of islet cell tumors. Surgery 120: 1051-1054. [Crossref]
- Dedieu A, Rault A, Collet D, Masson B, Sa Cunha A (2011) Laparoscopic enucleation of pancreatic neoplasm. Surg Endosc 25: 572-576. [Crossref]
- Sweet MP, Izumisato Y, Way LW, Clark OH, Masharani U, et al. (2007) Laparoscopic enucleation of insulinomas. Arch Surg 142: 1202-1204. [Crossref]
- Machado MA, Makdissi FF, Surjan RC, Machado MC (2009) Laparoscopic resection of uncinate process of the pancreas. Surg Endosc 23: 1391-1392. [Crossref]
- Rotellar F, Pardo F, Benito A, Martí-Cruchaga P, Zozaya G, et al. (2011) Laparoscopic resection of the uncinate process of the pancreas: the inframesocolic approach and hanging maneuver of the mesenteric root. Surg Endosc 25: 3426-3427. [Crossref]
- Tsai FJ, Lee JY, Chang YT (2011) Laparoscopic resection of a giant solid pseudopapillary neoplasm of uncinate process of the pancreas in a child. J Laparoendosc Adv Surg Tech 21: 979-982. [Crossref]
- Costi R, Randone B, Mal F, Basato S, Lévard H, et al. (2013) A critical appraisal of laparoscopic pancreatic enucleations: right-sided procedures (Pancreatic Head, Uncus) are not mini-invasive surgery. Surg Laparosc Endosc Percutan Tech 23: 524-531.
- Heeger K, Falconi M, Partelli S, Waldmann J, Crippa S, et al. (2014) Increased rate of clinically relevant pancreatic fistula after deep enucleation of small pancreatic tumors. Langenbecks Arch Surg 399: 315-321. [Crossref]
- Brient C, Regenet N, Sulpice L, Brunaud L, Mucci-Hennekine S, et al. (2012) Risk factors for postoperative pancreatic fistulization subsequent to enucleation. J Gastrointest Surg 16: 1883-1887. [Crossref]




