We evaluated the 7, 12- dimethylbenz (a) anthracene (DMBA) induced mouse skin tumor development as a function of time and its control by C-Phycocyanin (C-PC, a phycobiliproteins from Spirulina). Oral administration of C-PC inhibited the DMBA induced skin tumor development in term of number and the size of tumors. C-PC administration also prevented the DMBA caused molecular alterations in terms of mutp53, Bcl-2, Cdc25A and p27, Bcl-2 at different end points. There were no tumors at 1 week or 1 month after DMBA exposure but appeared after 4 months which were prevented in presence of C-PC. We show that C-PC prevented the skin tumor development possibly due to its involvement in the inhibition of DMBA mediated p53 mutation, increasing apoptosis and modulating cell cycle regulators in terms of higher expression of Bcl-2 and Cdc25A or p27genes. These results make the basis of detailed study of C-PC as a potentially safe antitumor agent.
C-Phycocyanin, Skin tumor, 7, 12- dimethylbenz (a) anthracene, Bcl-2, Cdc25A, mut p53, p27
There has been a considerable interest in the use of marine natural products for the chemopreventive activity against skin tumor development [1,2]. C-Phycocyanin (C-PC) is a phycobiliprotein from Spirulina, which is water soluble, blue colored and gives red fluorescence. C-PC has been reported to have antioxidant, anti-inflammatory, neuroprotective [3,4], hepatoprotective [5] effects and induces apoptotic events in various cell lines [6-8]. The spectrum of chemotherapeutic agents causing apoptosis has expanded progressively; we studied the effect of oral administration of food grade C-PC on some of the hallmark events of apoptosis and cell cycle regulators in mouse skin, a very well established model system as compared to other in–vivo models [9]. 7, 12-dimethylbenz (a) anthracene (DMBA) is commonly employed to induce skin tumors in mice. Mouse skin tumors can be induced using repeated topical applications of sub carcinogenic dose of DMBA in Swiss albino mice [10]. Present study deals with the effects of topical application of C-PC on some of the selective and critical events of apoptosis after DMBA exposure.
Significantly higher incidence of mutated p53 overexpression has been shown in metastatic melanomas than in primary melanomas [11], suggesting that loss of p53 function promotes cancer progression. p53 is essential in maintaining genomic integrity through their ability to block DNA replication in response to DNA damage. p53 also have a direct role in DNA repair [12] . Upregulation of p53 result in a transient G1 arrest allowing cells to repair DNA damage before resuming the cell cycle [13]. There is also a p53 dependent pathway whereby cells undergo apoptosis for self repair [14].
In skin, overexpression of Bcl-2 in murine keratinocytes led to an increased susceptibility to chemically induced tumors [15]. Like Bcl-2, Bcl-XL is also an anti apoptotic gene involved in the malignant conversion of chemically induced skin papilliomas [16]. The cell division cycle 25 (Cdc25) is a member of Cdc25 family of phosphatases consisting Cdc25A, Cdc25B and Cdc25C which regulate progression through the cell cycle and maintain genomic stability in response to DNA damage [17]. Earlier we reported the isolation and the effects of C-PC on early changes by skin tumor promoter [18]. Now, we show the chemopreventive effect of C-PC on chemically induced complete tumorigenesis and its basis in terms of cell cycle and apoptotic regulators at early or late stages after the DMBA exposure.
Animals
Female Swiss mice (4-6 wk old) were maintained in 12 h light/dark cycle, fed on synthetic pellet diet and water ad libitum at CSIR- IITR, Lucknow. Synthetic diet , procured from M/S Ashirwad Pvt. Ltd., Chandigarh, India, consisted of 21.53 % protein, 5.24% fat, 5.3% crude fibre, 57.59% carbohydrate and 363.64 kcal/100g caloric value. The transportation, experimentation and care of the animals were performed in compliance with the relevant laws and institutional guidelines.
Study was approved by and animals were handled according to the norms of Institutional Animal Ethics Committee (IAEC, CSIR-Indian Institute of Toxicology Research, Lucknow, India).
Chemicals
DMBA was purchased from Sigma Co. St. Louis, MO USA. Dry Spirulina powder (for C-phycocyanin purification) was purchased from Ambe phytoextract, Delhi, India. Reagents for reverse-transcription PCR (RT-PCR) were from Bangalore genie and rest of the chemicals were from the local sources and were of analytical grade.
C-Phycocyanin
Food grade C-phycocyanin was purified (1.66, purification ratio, A620/A280) from Spirulina powder (from Ambe Phytoextract, Delhi, India) in our laboratory as described earlier [18].
Chronic animal bioassay
DMBA induced mouse skin tumorigenesis protocol was followed as described [19] and chemopreventive potential of oral C-PC was evaluated .Animals were shaved on the back in the interscapular region and were divided in four groups each consisting of 15 animals. Animals in each group were kept in three separate cages (three animals/cage). Group 1- acetone 100 µl; Group 2 -0.1% C-PC ,ad libitum in drinking water; Group 3- DMBA,5 ug in 100 ul acetone, topically ; Group 4- DMBA and C-PC concomitantly . DMBA in acetone was applied topically twice a week in presence or absence of 0.1% C-PC in drinking water. Animals from each group were sacrificed at 1week, one or 4 months after the treatment. Selection of C-PC dose was based on the earlier reports [20,21]. A portion of skin was fixed in 4% buffered formalin for histopathological analysis as described [22] and a portion of skin was processed for the molecular analysis. 5 μm H&E stained sections were examined using Leica DFC 295 camera under Leica DM 1000 microscope at the magnification of 100X.
Protein analysis by immunoprecipitation and Western Blotting
Epidermal extract was prepared and proteins were separated as described [22]. Protein of interest was detected by probing with the primary antibody for Bcl-2, Cdc25A, p27 polyclonal antibody (Santa Cruz biotechnology), mutant p53 (Boehringer Mannheim, Germany). Proteins were detected using peroxidase-conjugated appropriate secondary antibody (Bangalore Genei, India) and signals were visualized by Chemilumniscence HRP detection system (Millipore) on Versa Doc (Bio-Rad) and band intensity was quantified by Syngene gene tools. Membranes were stripped and re-probed with β-actin antibody (Sigma Chemical Co. USA) to serve as reference. Mutant p53 was analyzes by immunoprecipitation as per the protocol described earlier [22].
mRNA analyses by Reverse-Transcriptase PCR (RT-PCR)
RNA extraction and cDNA preparation was done as described [22] and amplification was done using mRNA specific primers (MWG Bio Tech, Germany) for specific genes (Table 1). Ampli Taq DNA polymerase, (Ambion Co.) was used for PCR with the hot start at 95°C for 10 min, 35 cycles (95°C for 60s, annealing for 60s and 72°C for 60s) was done with a final extension at 72°C for 4 min. PCR products were resolved on 1.5% agarose gel containing ethidium bromide and quantification was done using Gene Tool Syngene software.
Table 1. Nucleotide sequences for RT-PCR primers.
Gene |
Nucleotide sequences |
Annealing temp. (°C) |
Product size (bp) |
Mut p53 |
F- 5’-ATGACTGCCATGGAGGAGTC-3’
R- 5'- CTCGGGTGGCTCATAAGGTA-3' |
60 |
663 |
Bcl-2 |
F- 5’- AGCCCGTGTTTGTAATGGAG -3’
R- 5’- CACAGCCTTGATTTTGCTGA -3’ |
58 |
476 |
Cdc25A |
F 5’AGAACCCTATTGTGCCTACTG3’
R 5’TACTCATTGCCGAGCCTATC 3’ |
58 |
124 |
p27 |
F 5’CAGAATCATAAGCCCCTGGA3’
R 5’TCTGACGAGTCAGGCATTTG 3’ |
58.52 |
224 |
β-actin |
F 5’-TGTGATGGTGGGAATGGGTCAG-3’
R 5’-TTTGATGTCACGCACGATTTCC-3’ |
60 |
514 |
Isolation of C-PC
C-PC was purified form dry Spirulina powder in our lab as published earlier [18]. Concentration of C-PC in this solution and its purity was determined by its spectrophotometric analysis. C-PC purity ratio (A620/A280) was 1.66 which was in the range of food grade C-PC. This was used for feeding to animals.
Effect of C-PC on DMBA caused mouse skin tumor development at 1, 4 or 16 weeks
The remarkable observation was the significantly reduced and suppressed growth of tumors in presence of C-PC as compared to that of DMBA alone at 16 weeks. As per the physical appearance of the tumors, there was a drastic difference in the size of tumors between DMBA treated and rest of the experimental groups. Initially, tumor appeared as a minute wart like growth and was firmly attached to the bases with fragile tops. Tumors, encountered in the different groups, were mainly benign in nature and some tumors remained like acne till the end of the experiment. DMBA application did not cause any tumor development at 1 or 4 weeks after application. At 16 weeks, number of tumors was 12.8 tumors per animal in DMBA treated group of mice .Treatment with C-PC delayed the onset of tumor and inhibited the tumor formation by DMBA showing 4.25 tumor per animal in DMBA treated animas drinking 0.1% C-PC. This resulted into 64.83% protection of tumor development by C-PC. Tumor bearing animals and its analysis at 16 weeks is shown in Figure 1A & B and Table 2. Acetone exposed skin remained unaltered but severe hyperplasia and dysplasia at four weeks after treatment and well-defined tumors at 16 weeks in histological analysis. Slight hyperplasia or dysplasia and few small pappilomas were there in DMBA exposed mice drinking C-PC at 4 or 16 weeks (Figure 1C, magnification 100X).
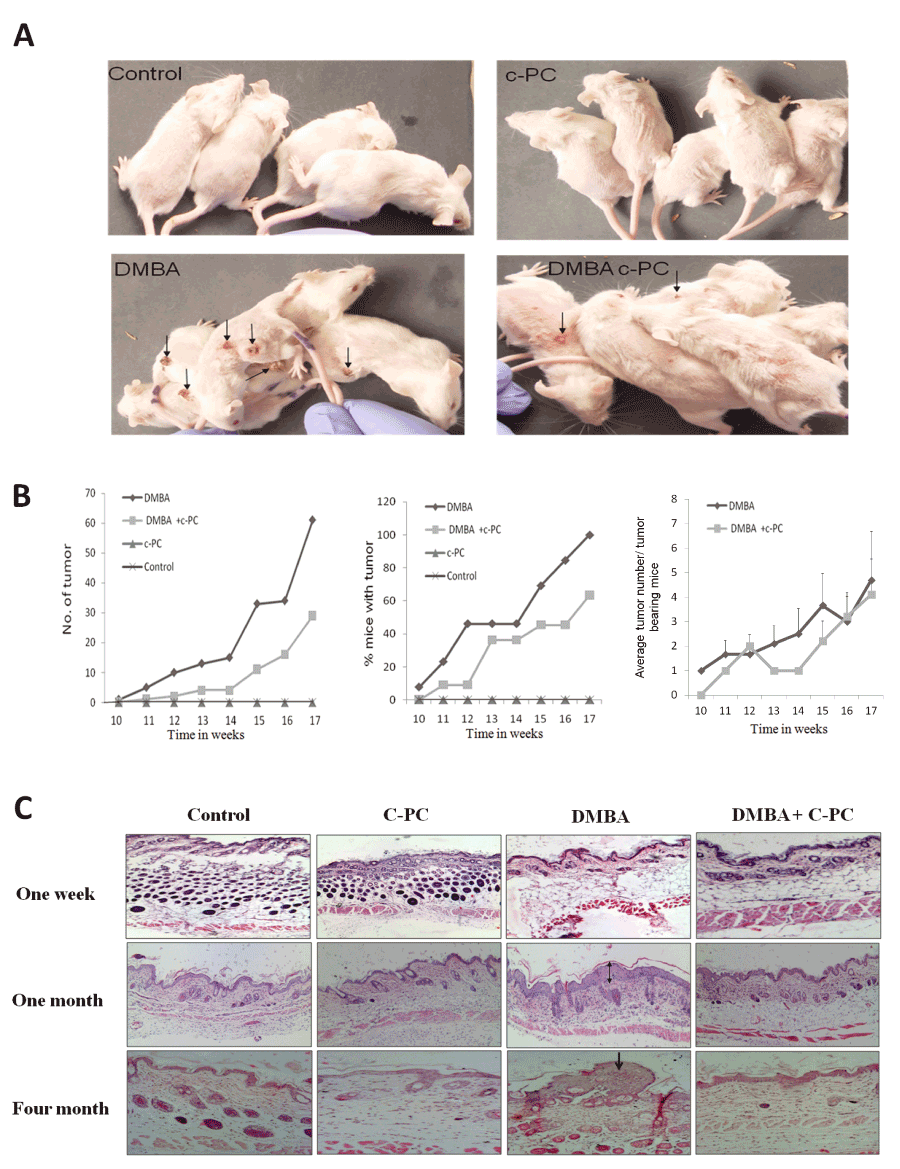
Figure 1. Status of mouse skin tumors.Tumors (A) and quantitative analysis (B). Skin tissue analyzed by histopathology at 1wk, 1 or 4 months (C).Hyperplasia is indicated by double and tumor by single arrow.
Table 2. Status of tumors at the end of four months of exposure.
Treatment |
No. of Tumors |
Tumor / animal |
Tumor bearing animals
/ total no. of animals |
DMBA |
61 |
4.69 |
13/13 |
DMBA + C-PC |
29 |
2.9 |
7/11 |
% Protection |
52.45 |
38.16 |
36.37 |
Analysis of mutant p53
DMBA application upregulated the expression of mutp53 at 1, 4 or 16 weeks at the protein level. mtp53 mRNA was not affected at 1 week but at 4 or 16 week showed upregulation. Administration of C-PC along with DMBA significantly lowered expression of mtp53 at the level of protein or mRNA suggesting the inhibition of DMBA induced mutation in p53 gene by C-PC (Figure 2, A-D).
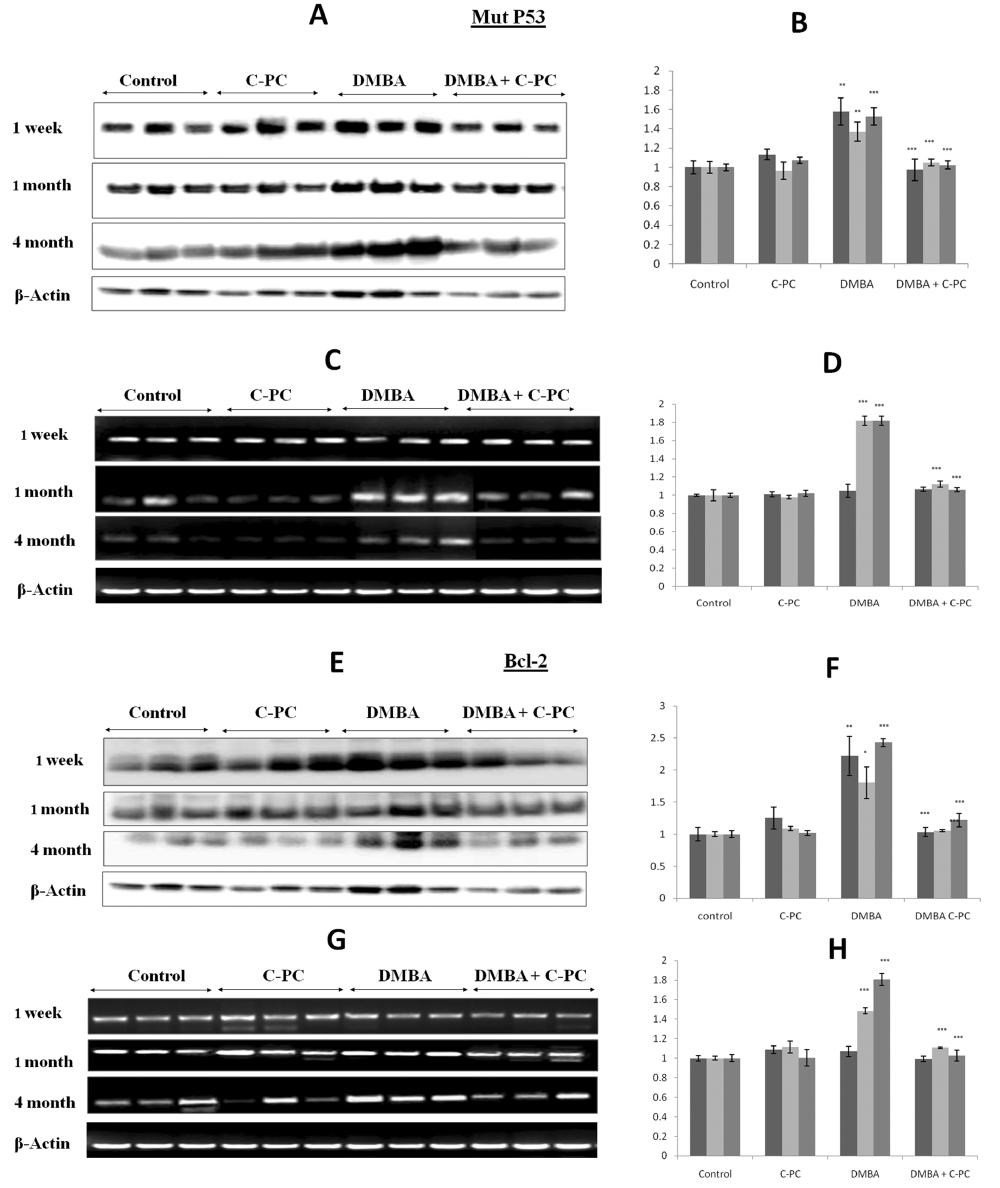
Figure 2. Mutp53 and Bcl-2 expression.Qualitative / quantitative analysis of mut p53 protein is shown in A / B and mRNA analysis is shown in C /D. Qualitative / quantitative analysis of Bcl-2 protein is shown in E / F. and Bcl-2 mRNA is shown in G / H. Bars represent standard error. ** P<0.01, *** P<0.001.
Evaluation of status of Bcl-2
Bcl-2 was upregulated after DMBA application at 1, 4 or 16 weeks. Bcl-2 protein levels appeared to be more or less same at all the time points but at mRNA level Bcl-2 expressions appeared to be a function of time. Administration of C-PC to the DMBA exposed animals prevented the upregulation of Bcl-2 in a very significant manner. Bcl-2 expression was not affected in mice receiving C-PC alone at any time point of DMBA exposure. Overall, C-PC intake prevented the DMBA caused Bcl-2 upregulation by 50% (Figure 2, E-H).
Evaluation of Cdc25A status
DMBA application up regulated the Cdc25A expression at 1, 4 or 16 weeks at the protein and at the mRNA level as compared to untreated control. The upregulation was more pronounced at the mRNA level as compared to protein level. Though this upregulation was slightly more at 16 weeks not much difference was seen between the exposure times. Presence of C-PC along with DMBA exposure prevented the upregulation of Cdc25A protein or mRNA at all the time points in a very significant manner and maintained the levels to the normal controls (Figure 3, A-D).
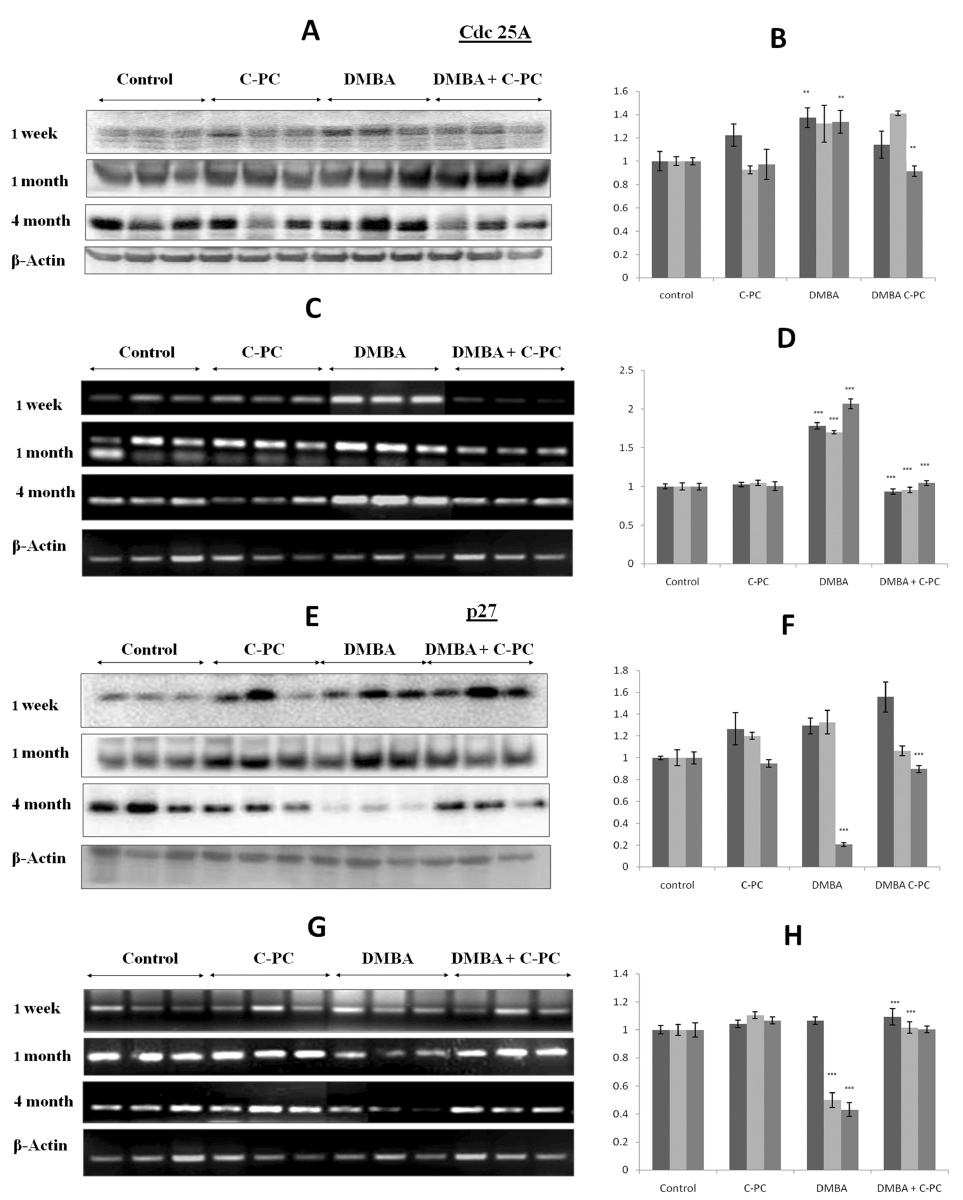
Figure 3. Cdc25A and p27 expression.Qualitative / quantitative analysis of Cdc25A protein is shown in A / B and mRNA is shown in C /D. Qualitative and quantitative analysis of p27 protein is shown in E / F and mRNA is shown in G / H. Relative band volume density is with respect to control group. Bars represent standard error. ** P<0.01, *** P<0.001.
Analysis of p27
In order to see the effects of C-PC on cell cycle regulation we assessed the levels of cyclin-dependent kinase inhibitor p27. DMBA application could not alter the p27 at protein or mRNA level at 1 week. p27 protein was also not affected by DMBA at 4 weeks but p27 mRNA was down regulated very significantly. At 16 weeks, the time of tumor appearance, p27 was drastically downregulated both at the protein and mRNA level. Administration of C-PC to the DMBA exposed animals tried to prevent the DMBA mediated down regulation of p27 and maintained to the normal levels (Figure 3, E-H).
Several reports are available on the chemopreventive potential of different compounds for skin tumorigenesis [23]. However, to translate these chemopreventive agents into chemotherapeutic compounds, their exact mechanism of action must be delineated. Earlier we had shown the effect of C-PC on the TPA induced molecular changes in an acute study [18]. This suggested that C-PC, like other chemopreventive agents, may act on one or several steps (initiation, promotion, progression) of carcinogenesis. DMBA-induced mouse skin carcinogenesis is commonly used to test chemo-preventive efficacy of medicinal plants and their constituents [23,24].
Here we showed that the oral administration of C-PC significantly prevented the formation of well differentiated squamous cell carcinomas in DMBA treated animals by monitoring the protection in term of tumors per animal and an average tumor area per animal. Chemopreventive effect could be due to the antioxidant property of C-PC. C-PC has also been shown to exhibit the anti oxidant property [3,4] and also inhibition of pro-inflammatory cyclooxygenase-2 (COX-2) [25] that is up-regulated during inflammation and cancer. Various mechanisms of actions have been reported for the chemopreventive agents that include antiproliferative efficacy, induction of apoptosis, and cell cycle arrest, anti lipid peroxidative and antioxidant effects [23]. Likewise, we showed the involvement of cell cycle and apoptosis regulators like p53, Bcl-2, Cdc25A and p27 to decipher the basis of antitumor effects of C-PC.
We showed the higher level of mutated p53 at all time points in DMBA treated group which was prevented in presence of C-PC and the upregulation of apoptotic regulator Bcl-2, cell cycle regulator Cdc25A and p27 by DMBA as a function of time. C-PC administration tried to maintain the normal status in DMBA exposed mice. We provide with more data to support the hypothesis that C-PC exerts its tumor inhibitory effect by inducing the apoptotic pathways. High level of p53 reduces the Bcl-2 expression leading to the induction of apoptosis [26]. In the similar line of action, we showed that C-PC administration prevented the DMBA caused Bcl-2 upregulation before or after the onset of tumors. This suggested that C-PC might exerts its anti tumor effect by inhibiting the antiapoptotic protein Bcl-2 as antitumor agents inhibit Bcl-2 [27].
Though, a key regulator of cell cycle progression [17], little is known about the role of Cdc25A in skin carcinogenesis. We have presented the data on the status of Cdc25A before and after the onset of tumors. Cdc25A mRNA showed significant upregulation which was protected by the presence of C-PC. Alterations in Cdc25A were maintained from start to the finish of tumor development. Cdc25A could be the better target for the antitumor agents as it is altered before the tumor is developed. Our study is supported by other reports showing that Cdc25A expression is significantly increased in different human cancers [28-30]. Reports on the role of Cdc25A phosphatase in DNA damage checkpoints and our results showed that C-PC administration inhibited the DMBA induced tumorigenesis by inhibiting the Cdc25A. We feel that inhibition of DMBA induced Cdc25A in the skin would help for improved repair of DNA damage and decreasing skin tumorigenesis by C-PC. These data also suggest that Cdc25A may be an important target for cancer control.
Cdc25A status suggested that C-PC exerted its antitumor tumor effects by altering the cell cycle regulators in terms of another cell cycle suppressor gene, p27, a negative regulator of Cyclin/CDK complexes and a potential target in cancer prevention studies [31]. Various nutritional and chemopreventive anti-cancer agents up-regulate expression of p27 in pre-neoplastic cells [32]. Here also p27 was down regulated when tumor appeared and prevented by C-PC. p27 was not altered much at early stage possibly due to the lack of modulation of p27 associated events.
Study of the chemopreventive effects of C-PC in DMBA exposed mouse skin has not been reported earlier. Our results showed that the DMBA caused alterations much before the onset of tumors were protected by the presence of C-PC. C-PC could be exerting its antitumor effects through the inhibition of cell cycle progression and induction of the pro- apoptotic events. Since C-PC was able to prevent the molecular changes which were involved in tumor development and also the tumor development, it may be put among the potential agents to be exploited for the cancer control.
There is no conflict of interests among the authors.
This work was supported by the Indian Council of Medical Research, New Delhi. India.
Authors thank the Director, CSIR-Indian Institute of Toxicology Research, Lucknow, for his support and Mr. Chandra Shekhar Yadav for his assistance in histological work.
- Fahmy H, Zjawiony JK., Konoshima T, Tokuda H, Khan S, et al. (2006) Potent skin cancer chemopreventing activity of some novel semi-synthesis cembranoids from marine sources. Mar Drugs 4: 1-9. [Crossref]
- Zhang X, Kundoor V, Khalifa S, Zeman D, Fahmy H, Dwivedi C (2007) Chemopreventive effects of sarcophine-diol on skin tumor development in CD-1 mice. Cancer Lett 253: 53-59.
- RomayCh, González R, Ledón N, Remirez D, Rimbau V (2003) C-phycocyanin: a biliprotein with antioxidant, anti-inflammatory and neuroprotective effects. Curr Protein PeptSci 4: 207-216. [Crossref]
- González R, Rodríguez S, Romay C, Ancheta O, González A, et al. (1999) Anti-inflammatory activity of phycocyanin extract in acetic acid-induced colitis in rats. Pharmacol Res 39: 55-59. [Crossref]
2021 Copyright OAT. All rights reserv
- Vadiraja BB, Gaikwad NW, Madyastha KH (1998) Hepatoprotective effects of C-phycocyanin: protection for carbon tetrachloride and R-(+)-pulegone -mediated hepatotoicity in rats. BiochemBiophys Res Commun 249: 428-431.
- Pardhasaradhi BVV, Ali AM, Kumari AL, Reddanna P, Khar A (2003) Phycocyanin-mediated apoptosis in AK-5 tumor cells involves down-regulation of Bcl-2 and generation of ROS. Mol Cancer Ther 2: 1165-1170.
- Subhashini J, Mahipal SV, Reddy MC, Mallikarjuna Reddy M, Rachamallu A, et al. (2004) Molecular mechanisms in C-Phycocyanin induced apoptosis in human chronic myeloid leukemia cell line-K562. BiochemPharmacol 68: 453-462. [Crossref]
- Li B, Gao MH, Zhang XC, Chu XM (2006) Molecular immune mechanism of C-phycocyanin from Spirulinaplatensis induces apoptosis in HeLa cells in vitro. BiotechnolApplBiochem 43: 155-164. [Crossref]
- Boutwell RK (1984) Some biological aspects of skin carcinogenesis. ProgExp Tumor Res 4: 207-250.
- Vellaichamy L, Balakrishnan S, Panjamurthy K, Manoharan S, Alias LM (2009) Chemopreventive potential of piperine in 7,12-dimethylbenz[a]anthracene-induced skin carcinogenesis in Swiss albino mice. Environ ToxicolPharmacol 28: 11-18. [Crossref]
- Grant SW, Kyshtoobayeva AS, Kurosaki T, Jakowatz J, Fruehauf JP (1998) Mutant p53 correlates with reduced expression of thrombospondin-1, increased angiogenesis, and metastatic progression in melanoma. Cancer Detect Prev 22: 185-194. [Crossref]
- Smith ML, Chen IT, Zhan Q, O'Connor PM, Fornace AJ Jr. (1995) Involvement of the p53 tumor suppressor in repair of u.v.-type DNA damage. Oncogene 10: 1053-1059. [Crossref]
- Kuerbitz SJ, Plunkett BS, Walsh WV, Kastan MB (1992) Wild-type p53 is a cell cycle checkpoint determinant following irradiation. ProcNatlAcadSci USA 89: 7491-7495. [Crossref]
- Liebermann DA, Hoffman B, Steinman RA (1995) Molecular controls of growth arrest and apoptosis: p53-dependent and independent pathways. Oncogene 11: 199-210. [Crossref]
- Rodríguez-Villanueva J, Greenhalgh D, Wang XJ, Bundman D, Cho S, et al. (1998) Human keratin-1.bcl-2 transgenic mice aberrantly express keratin 6, exhibit reduced sensitivity to keratinocyte cell death induction, and are susceptible to skin tumor formation. Oncogene 16: 853-863. [Crossref]
- Pena JC, Rudin CM, Thompson CB (1998) A Bcl-xL transgene promotes malignant conversion of chemically initiated skin papillomas. Cancer Res 58: 2111-2116. [Crossref]
- Boutros R, Lobjois V, Ducommun B (2007) CDC25 phosphatases in cancer cells: key players? Good targets? Nat Rev Cancer 7: 495-507. [Crossref]
- Gupta NK, Gupta KP (2012) Effects of C-Phycocyanin on the representative genes of tumor development in mouse skin exposed to 12-O-tetradecanoyl-phorbol-13-acetate. Environ ToxicolPharmacol 34: 941-948. [Crossref]
- Gupta KP, Singh J, Bharathi R (2003) Suppression of DMBA-induced mouse skin tumor development by inositol hexaphosphate and its mode of action. Nutr Cancer 46: 66-72. [Crossref]
- Shih CM, Cheng SN, Wong CS, Kuo YL, Chou TC (2009) Antiinflammatory and antihyperalgesic activity of C-phycocyanin. AnesthAnalg 108: 1303-1310. [Crossref]
- Chun KS, Keum YS, Han SS, Song YS, Kim SH, et al. (2003) Curcumin inhibits phorbol ester-induced expression of cyclooxygenase-2 in mouse skin through suppression of extracellular signal-regulated kinase activity and NF-kB activation. Carcinogenesis 24: 1515-1524. [Crossref]
- Pandey M, Gupta KP (2011) Epigenetics, an early event in the modulation of gene expression by inositol hexaphosphate in ethylnitrosourea exposed mouse lungs. Nutr Cancer 63: 89-99. [Crossref]
- Manoharan S, Singh RB, Balakrishnan S. 2009. Chemopreventive mechanisms of natural products in oral, mammary and skin carcinogenesis: An Overview.The Open Nutraceuticals Journal.2:52-63.
- Youn BH, Yang HJ (2011) Chemoprevention of skin cancer with dietary phytochemicals. In: Yaguang Xi (ed) Skin cancer overview. InTech.
- Reddy CM, Bhat VB, Kiranmai G, Reddy MN, Reddanna P, et al. (2000) Selective inhibition of cyclooxygenase-2 by C-phycocyanin, a biliprotein from Spirulinaplatensis. BiochemBiophys Res Commun 277: 599-603. [Crossref]
- Dada MA, Chetty R, Biddolph SC, Schneider JW, Gatter KC (1996) The immunoexpression of bcl-2 and p53 in Kaposi's sarcoma. Histopathology 29: 159-163. [Crossref]
- Adhami VM, Aziz MH, Mukhtar H, Ahmad N (2003) Activation of prodeath Bcl-2 family proteins and mitochondrial apoptosis pathway by sanguinarine in immortalized human HaCaT keratinocytes.Clin Cancer Res9: 3176-3182. [Crossref]
- Xu X, Yamamoto H, Sakon M, Yasui M, Ngan CY, et al. (2003) Overexpression of CDC25A phosphatase is associated with hypergrowth activity and poor prognosis of human hepatocellular carcinomas. Clin Cancer Res 9: 1764-1772. [Crossref]
- Ito Y, Yoshida H, Nakano K, Kobayashi K, Yokozawa T, et al. (2002) Expression of cdc25A and cdc25B proteins in thyroid neoplasms. Br J Cancer 86: 1909-1913. [Crossref]
- Broggini M, Buraggi G, Brenna A, Riva L, Codegoni AM, et al. (2000) Cell cycle-related phosphatases CDC25A and B expression correlates with survival in ovarian cancer patients. Anticancer Res 20: 4835-4840. [Crossref]
- Taylor W, Mathias A, Ali A, Ke H, Stoynev N, et al. (2010) p27Kip1 deficiency promotes prostate carcinogenesis but does not affect the efficacy of retinoids in suppressing the neoplastic process. BMC Cancer 10: 541.
- Eto I (2006) Nutritional and chemopreventive anti-cancer agents up-regulate expression of p27Kip1, a cyclin-dependent kinase inhibitor, in mouse JB6 epidermal and human MCF7, MDA-MB-321 and AU565 breast cancer cells. Cancer Cell Int 6: 20. [Crossref]



