Vasopressin is one of the main regulation hormones of fluid homeostasis. Secretion of vasopressin from the pituitary is mainly triggered by small changes in plasma osmolality, which leads to reabsorption of free water in the collecting ducts of the kidney, and normalization of the osmolality. Studies have shown that there’s a circadian rhythm of the hormone, with higher levels throughout the night. Therefore, urine production is reduced during the night, and people don’t have to void during sleep time. A low nocturnal level of vasopressin is thought to be one of the main causes of in increased nocturnal urine production, leading to nocturnal polyuria and nocturia. These conditions have an underestimated effect on quality of life, and are an important cause of associated morbidities. Therapy consists of substitution of vasopressin with the synthetic analogue desmopressin. But, not all patients react to desmopressin, and treatment of global polyuria of unknown origin should be avoided. This makes the vasopressin a potential biomarker in the work-up of patients with nocturia and nocturnal polyuria, but half-life of the hormone is short, making it difficult to measure. Copeptin, the C-terminal part of pre-pro-vasopressin is co-secreted in equimolar concentration with vasopressin. In contrast to vasopressin itself, it is a much more stable peptide, and measurement is less cumbersome and expensive.
Following the hypothesis that nocturnal polyuria patients have low vasopressin at night, we expect these patients to have low copeptin levels at night through the early morning, but normal levels during the daytime. This in contrast to patients with polyuria-polydipsia syndrome, where we expect low values throughout the day and night. Patients with nocturia due to a reduced bladder capacity would have normal values, since they have no deficiency in their circadian rhythm of vasopressin. Therefore, copeptin may be potentially used to determine the ideal candidate for desmopressin therapy in nocturia. As there are a lot of factors that influence the levels of vasopressin, and thus copeptin, we set up this study to research the daytime variation of copeptin in healthy patients and to determine which conditions (fasting, following a meal, …) and timing (morning, daytime,…) influences copeptin measurement.
In September 2015, 25 volunteers were included in this observational prospective study. A blood sample was taken at four different time points during the day for measurement of copeptin. The first (T1) was a sober morning sample, at this time we also measured serum sodium, creatinine and osmolality. The second sample (T2) was taken between 10:00 and 11:30 after breakfast, the third (T3) between 13:00 and 14:30 after lunch, and the last (T4) between 15:30 and 17:00. At time of each blood sample a urine sample was provided as well. Volume of each sample had to be noted, and osmolality, sodium and creatinine were measured on each of these urine samples, to calculate the renal clearance of these substances. Plasma samples were centrifuged at 4°C, and stored at -80°C until time of measurement. Copeptin levels were expressed in pmol/L. The lower detection limit was 0,9pmol/L and the functional assay sensitivity (<20% interassay coefficient of variation) was less than 2pmol/liter. Medians and interquartile ranges were recorded as descriptive statistical parameters. Mann-Whitney U and Kruskal-Wallis analysis of variance and Spearman's correlation analysis were used for statistical analysis. A p-value <0.05 was considered statistically significant.
Median age of the population was 27 years (IQR: 25 – 31,5, range: 18 – 53), and 50% of participants were female. None of the patients had 2 or more nocturia episodes per night. The sober morning copeptin concentration was higher than the three other samples (p = 0.032) (See Table 1 and Fig. 1). One patient who fainted during blood sampling was excluded from analysis, his copeptin concentration at that time was 456,8 pmol/L, which resembles values from acute sepsis.
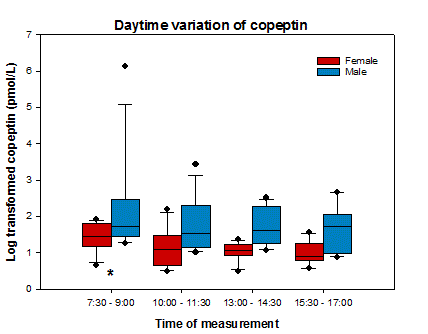
Figure 1. Daytime variation of copeptin.
Table 1. Daytime variation of copeptin
| |
|
T1 *
(7 – 9 am)
(sober)
|
T2
(10 – 11 am)
|
|
T3
(1 – 2 pm)
|
|
T4
(4 – 5 pm)
|
|
P –value
(KW)
|
|
Copeptin (pmol/L)
|
|
5,04
(4,01 – 6,51)
|
3,48
(2,78 – 5,64)
|
|
3,42
(2,90 – 5,53)
|
|
3,37
(2,41 – 6,05)
|
|
0.032
|
Values are depicted as median (interquartile range)
*copeptin concentration at T1 was significantly higher than the other 3 samples
Furthermore, we confirmed lower copeptin levels in females (3,13 pmol/L (IQR 2,36 – 4,19)) compared to males (5,14 pmol/L (IQR 3,64 – 8,55)) (p < 0.001). A positive correlation was found between copeptin and urinary osmolality (p < 0.001), and a negative correlation with diuresis rate
(p < 0.001) and FWC (p = 0.017). We suspected copeptin to be mostly correlated with the urinary parameters of the urinary sample produced at that time, or the following one, but this proved not to be true for all parameters. In this analysis no correlation was found between copeptin and plasma osmolality.
We can conclude that daytime variation in controls is limited, with slightly, but significantly higher values in the morning before food and fluid intake. This can be explained because vasopressin and thus copeptin, rises during the night, and can be expected to remain high in the morning before eating or drinking. We have to note that this a very young population, not characteristic for the population that mostly presents with nocturnal polyuria. When we extrapolate these results to a nocturic population, we can expect lower sober morning values in patients with global polyuria and nocturnal polyuria due to a deficiency in the circadian rhythm of vasopressin. Patients with reduced bladder capacity should have normal values. Random daytime samples, or simply a frequency-volume chart can differentiate between global and nocturnal polyuria.
In this study we did not take a late evening or nighttime blood sample, since we reasoned there is no immediate clinical use, as it is not feasible for patients to visit the outpatient clinic and have their blood taken late at night. Although there are advantages in measuring in late evening sample, not only for scientific knowledge, but also in the development of a home self-diagnostic tool, where patients could measure their copeptin levels in the same manner as glycemic control. Therefore, we did include a late evening sample in a follow-up study, where we repeat this same study, in a nocturia/nocturnal polyuria population.
Furthermore, conditions of blood sampling for copeptin measurement has to be standardized if we want to do further research on the use of copeptin in nocturia, also in early morning samples. Exercise and water intake are two important factors that can influence the copeptin concentration. We suggest blood sampling after a short resting period, for example 20 minutes. Fluid intake before early morning sampling should not be allowed. On the other hand, when patient water load before bedtime, vasopressin levels will be lower than normal. This is something we have to take into account for the possible use of evening samples, and can be a major confounding factor.
Copeptin is a promising parameter, but influenced by many factors that need to be evaluated before concluding it is a valuable tool for the prescription of desmopressin. We suggest using sober early morning samples in further nocturia research.

