Background: Chemoradiation toxic mucositis (CRTM) limits optimal cancer treatment, increases morbidity, the cost of care and incidence of premature cancer deaths. Complete prevention or rapid sustained elimination can provide significant health economic benefit. However for 40 years prior to 2013, there have been no meaningful therapeutic advances. High potency polymerized cross-linked sucralfate (HPPCLS) has been associated with prevention and rapid sustained elimination of toxic mucositis. This case series of seven patients highlights the several implications of these unexpected outcomes.
Method: Concise review of the impact of toxic mucositis, its morbidity, costs and mortality. Provide case synopsis of seven patients treated with HPPCLS, four to reverse the complication and three to prevent its onset. Discuss the implications of findings on theoretical understanding on toxic mucosits as embodied in 5 phase model, on the costs of care, morbidity and mortality of cancer treatment patients.
Results/Key observations: Patients with CRTM of Grade 2-4 oral and GI tract from 10 different anti-neoplastic regimens, including three immunotherapeutics, experienced rapid (2-3 days) reversal of CRTM using HPPCLS. Reversal was sustained through continued use of HPPCLS, despite ongoing chemoradiation. CRTM was prevented by HPPCLS in three patient who were expected to develop grade 3-4 CRTM. The rate ratio of HPPCLS outcomes compared to historically expected outcomes was statistically significant with the outcomes attributed to the use of HPPCLS.
Implications and conclusion: Significant clinical outcomes attributed to HPPCLS have implications for impact on cost of care, patient morbidity and mortality. Additionally the 5 phase model for mucositis pathobiology may require modification to accommodate prevention and sustained elimination during ongoing exposure to mucotoxic cancer treatments.
chemoradiation mucositis, mucositis pathobiology, polymerized cross-linked sucralfate
Chemoradiation toxic mucositis is a gastrointestinal adverse reaction to non-surgical cancer treatment therapies. Its occurrence in the oropharynx, esophagus, small bowel and colon, often lead to regional organ dysfunction, namely – (a) the inability to eat, drink and swallow due to pain and ulceration in the upper GI tract, (b) treatment-induced nausea, vomiting and cramping in the small bowel [1], (c) colonic diarrhea with dehydration and (d) intestinal febrile bacteremia due to inflammation-associated breech of epithelial tight junctions [2-4].
Having no meaningful therapeutic options over the past 40 years, medical and radiation oncologists were forced to view toxic mucositis as a complication of treatment to be accepted, managed but never to be eliminated or prevented. Over this period of time, fifteen generations of medical oncologists and correspondingly six generations of radiation oncologists have been trained to accept toxic mucositis as an irreversible and unpreventable complication of chemoradiation. Overall, treatment-induced mucositis has been documented to occur in 37% of patients receiving chemotherapy (CT) [5,6], in 89.5% of head neck cancer patients (HNC) receiving radiation [7-9] and in 100% of patients undergoing human stem cell transplantation patient (HSCT) [10,11].
Thus in US, among the 1.6 million diagnosed with cancer annually [12,13], peer-reviewed actuarial data [14-17] indicate that approximately 382,990 patients require chemotherapy (CT), 59,340 patients with head and neck caner require radiation HNC and 20,875 patients undergo human stem cell transplantation (HSCT) [18,19], for a total of 463,205 cancer patients vulnerable to develop treatment-induced mucositis annually. Approximately 212,501 of them develop oropharyngeal and esophageal mucositis with an estimated 41,501 dying premature cancer deaths directly attributable to chemoradiation toxic mucositis. Besides the mortality and obvious morbidity suffered by patients, there are increased costs and resource utilization approaching $10.58 billion spent to temporize patients’ medical conditions sufficiently enough for them to endure the next dose or cycle of chemoradiation [9,20-27]. While mucositis-mediated intestinal febrile bacteremia associated with HSCT contributes 3,412 annual deaths, the remaining 38,089 deaths arise from mucositis-dependent unplanned treatment interruptions and doses reductions lower [28,29] which unavoidably lowers the “kill dose intensity” required for optimal survival and remission. Lowered dose intensity prompts early disease recurrence and lowered 5 year survival [28,30-35].
Reported here are seven patients selected from a post-approval mucositis registry established to document oncologist’s use of FDA approved HPPCLS (ProThelial) and patient outcomes. There six males and 1 female. In four patients (Table 1) the intent of HPPCLS treatment was reversal of chemo-radiation toxic mucositis, while in three patients (Table 2) treatment intent was prevention of onset of mucositis. These patients were selected for the range of cancer treatment regimens giving rise to mucositis throughout the GI tract and for the two intents of management - reversal and prevention. All patients of the registry had similar outcomes reported elsewhere [36]. Mucositis was graded using the World Health Organization (WHO) scale for oral mucositis [37] and the European Organization for Research and Treatment of Cancer/Radiation Therapy Oncology Group (EORTC/RTOG) scale for gastrointestinal mucositis [38].
Patient 1: Reported previously [39] a 43 yo male with advanced head and neck squamous cell carcinoma, previous smoker, drinker with an unknown human pampiloma virus serology, required surgical debulking of the lesion and nodal dissection, and concurrent chemo-radiation comprised 6 weekly infusions of paclitaxel and carboplatin with simultaneous daily radiation totaling 201 Gy (71Gy for base of tongue, 71Gy to the tumor mass with an additional radiation dose of 59Gy to regional nodes).
Two weeks into chemoradiation, he developed Grade 2 oral mucositis and Grade 2 alimentary mucositis with dysgeusia and xerostomia. One and half gram (1.5 gm) doses of PCLS (also referred to as HPS high potency sucralfate) in suspension form was swished and swallowed three times daily for 2 d, then twice daily.
Patient initiated Non-compliance. Inadvertently, this patient discontinued PCLS during cancer treatment, having forgotten to use it. Four days later both oral (Grade 2) and gastrointestinal mucositis (Grade 2) returned, prompting patient to resume PCLS. Without a loading regimen, patient resumed PCLS using 1.5 gram to swish and swallow twice daily. Both OM and GIM resolved within 2 days. Observed from this patient’s experience was that within 48 h of resuming PCLS, there was complete disappearance of oral mucositis lesions and tenderness with patient-reported disappearance of pain, nausea and diarrhea. Absence of both GI and oral mucositis was sustained, throughout continued high dose concomitant radiation, carboplatin and paclitaxel. The patient suffered no recurrence of mucositis, required no opiate analgesia and no tube-feeding to supplement his regular oral diet. Ageusia and xerostomia persisted due to radiation effects on salivary glands and taste receptors. No adverse reactions attributable to PCLS were reported or noted.
Patient 2 was a 49 yo male with Stage 4 squamous cell carcinoma of the head and neck (tonsils) receiving 6 weeks of local radiation combined with intravenous cetuximab sustained Grade 3 OM, Grade 2 GIM had reversal of OM in 2 to 3 days on PCLS and reversal of GIM in 1 day with the onset of normal bowel movements. This patient tolerated PCLS well and no adverse reactions attributable to PCLS were reported or noted.
Patient 3 was a 48 yo male with Stage 4 pancreatic adenocarcinoma receiving every 2 weeks infusion of Folfirinox (5-fluorouracil, folinic acid, irinotecan and oxaliplatin) sustained Grade 4 OM and was completely gastrostomy-tube dependent, with Grade 3 GIM experienced reversal of OM in 3 days on PCLS and reversal of GIM in 2 day with the onset of normal bowel movements. The patient who was able to eat a regular food diet after 4 weeks’ dependence on tube-feeding remarked that PCLS was a “miracle medication”. This patient tolerated PCLS well and no adverse reaction was reported.
Patient 4 was a 48 yo female with advance stage widely metastatic melanoma, primary being excised from mid-right back, she went on to developed metastasis to regional nodes, both lung pleura, stomach, adrenals and brain, with cerebral metastates requiring gamma knife surgery twice. She was treated with ipilimumab and nivolumab and developed Grade 3 oral mucositis and Grade 4 chemo-induced diarrhea associated with nausea, vomiting and difficulty swallowing. Oral mucositis completely reversed in 2 days as did her chemo-induced diarrhea. She was also placed on high dose prednisone due to development of tranaminitis of the liver. Prior to PCLS, she had been maintained on pantoprazole, ondansetron, and diphenoxylate-atropine without significant effect for weeks. Symptom control occurred shortly (2-3 days) following introduction of PCLS. Oral ulcerations and pain resolved. Post-prandial nausea, vomiting and crampiness diminished significantly (though not entirely) and frequent bouts of diarrhea were replaced with the normal bowel movements of formed stool. This patient tolerated PCLS well and no adverse reaction was reported.
Patients 5,6,7 for prevention of anticipated mucositis: The management of mucositis includes both prevention and reversal. One radiation oncologist in this series used HPPCLS to prevent toxic mucositis from occurring in patients with a high likelihood to require a surgical feeding tube for anticipated severe pharyngoesophageal mucositis, which would disrupt patients’s ability to swallow (eat or drink). These patients, presented previously [40], were all males age 93, 68 and 55 with squamous cell carcinoma of the base of the tongue and local metastatic spread to adjacent nodes that required surgical removal. All were previous smokers, their human papilloma viral serology was unknown. Using PCLS 3-5 days prior to commencing a 42-49 day course of radiotherapy, and continuing its use throughout treatment neither of these patients developed mucositis (Grade 1-4) and none required a surgical feeding tube.
HPPCLS outcomes and the statistical significance
Tables 1 and 2 provide the outcomes associated with the use of HPPCLS, 2-3 day rapid and sustained elimination in 4 and complete prevention in 3 patients. The size of the treatment effect and its reproducibility among these patients elevates to the significance of the evidence [41]. Rapid, complete and sustained reversal of a disease process at a rate that is 10 fold greater than otherwise expected is termed a positive Glasziou treatment effect [42] and qualifies HPPCLS as an effective therapeutic intervention. The difference in effect size between treatment and placebo is roughly 10-50 base points [43]. Therefore comparisons between placebo and any prospective intervention require control of biases that confound outcomes. Glasziou concluded that while randomized trials aptly and quantitatively discern a true treatment signal from noise of experimental bias, the magnitude of the treatment effect of certain interventions, can be so dramatic that experimental bias can be statistically ruled out as an explanation of the observed treatment effect. Glasziou [42] asserted that implausibly large associations, both between treatment and confounding factors and between confounding factors and outcome, are required to explain comparative response rates of 5-10. He concluded that rate ratios (obtained by comparing time to point of improvement between any prospective and standard intervention) that are beyond 10 reflect real treatment effects of the prospective intervention, even if confounding factors (experimental bias) were uncontrolled. In other words, the contributions of uncontrolled experimental bias would be trivial and non-determinative to the treatment outcome, having a p value of 0.05 or less. Oral mucositis in patients undergoing HSCT, a 42-49 day course of radiation or 4-6 cycles of chemotherapy can last 60 days, 84 days and 102 cumulative days respectively [44]. Reduction of mucositis to 2-3 days represents a Glasziou treatment effect ranging from 48 to 82 across all cancer treatment modalities; several folds greater than the required rate ratio of 10 to meet statistical significance.
Table 1. Four Case series of rapid & sustained elimination of chemo-radiation mucositis
Patient |
Age
Gender |
Institution/University |
Cancer |
Oncology Treatment |
Oral
Mucositis |
Gastrointestinal Mucositis |
Reversal of Ulceration |
Reversal of Painful Swallowing |
Reversal of Nausea, Cramps |
Reversal of Diarrhea |
|
1 |
43yoM |
Brown University & Boston University |
SCCHN |
Radiation+Carboplatin+Paclitaxel |
Grade 2 |
Grade 2 |
2 days |
2 days |
1 day |
1 day |
|
2 |
49yoM |
Midwestern |
SCCHN |
Radiation+Cetuximab |
Grade 3 |
Grade 2 |
2 days |
3 days |
1 days |
1 day |
|
3 |
48yoM |
Vanderbilt Ingram CaCtr |
Pancreatic Carcinoma |
Folfirinox (5-fluorouracil, folinic acid, irinotecan and oxaliplatin) |
Grade 4 |
Grade 3 |
3 days |
3 days |
2 days |
2 days |
|
4 |
48yoF |
UConn & Yale |
Metastatic Melanoma |
Ipilimumab
Nivolumab |
Grade 3 |
Grade 4 |
2 days |
2 days |
2 days |
2 days |
SCCHN: Squamous cell carcinoma of head and neck; Reversal: Mucositis elimination
Table 2. Three case series of complete prevention of chemo-radiation mucositis
Patient |
Age
Gender |
Cancer
Type |
Time from start of Cancer
Tx |
Institution |
Grade of
Mucositis |
Location of
Mucositis |
Therapeutic
Outcome |
5 |
93 yo male |
SCC Tongue |
0 weeks Radiation
|
Kansas
Rad Onc |
Anticipated Grade 3-4 |
100%Anticipated Requirement of
Feeding G-Tube |
G-Tube Averted
None Required While on ProThelial
Swallowed ProThelial™ |
6 |
55 yo male |
SCC Tongue |
0 weeks Radiation
|
Kansas
Rad Onc |
Anticipated Grade 3-4 |
100%Anticipated Requirement of
Feeding G-Tube |
G-Tube Averted
None Required While on ProThelial
Swallowed ProThelial™ |
7 |
68 yo male |
SCC Tongue |
0 weeks
Chemo+Radiation
|
Kansas
Rad Onc |
Anticipated Grade 3-4 |
100%Anticipated Requirement of
Feeding G-Tube |
G-Tube Averted
None Required While on ProThelial
Swallowed ProThelial™ |
SCC:Squamous cell carcinoma; Reversal: Mucositis elimination
Observation regarding cancer treatments’ mechanisms of action, anatomic location and severity of mucositis
In this seven-patient series, ten different anti-neoplastic treatments (Table 3) gave rise to symptomatic mucositis occurring in the oral cavity, pharynx, esophagus, small and large intestine. The severity of mucositis ranged from Grad 2 to Grade 4 using the WHO scale [37] for oral mucositis and the EORTC/RTOG scale for gastrointestinal mucositis [38].
Table 3. Agents used in case series causing toxic mucositis
Standard Radiation
Carboplatin
Paclitaxel
|
5-Fluorouracil
Folinic acid
Irinotecan
Oxaliplatin |
Cetuximab
Ipilimumab
Nivolumab |
Standard radiotherapy, the six-different traditional chemotherapeutic agents and three targeted immunotherapies led to mucositis in these patients through completely different mechanisms of action.
Table 3 shows the 10 different anti-neoplastic agents of this case series that caused toxic mucositis. Mucositis arise from tissue injury and each agent causes injury through different mechanisms. Radiotherapy damages cellular DNA, creating reactive oxygen species in cellular cytoplasm, with hypoxic and nutrient starved conditions within normal and tumor cells. The classic chemotherapy agents in this series (carboplatin, paclitaxel, 5-fluorouracil, folinic acid, irinotecan, oxaliplatin) have mechanism of actions targeting functional elements of the mitotic cycle in all dividing cells. while the immuno-chemotherapeutics (cetuximab, ipilimumab and nivolumab) have different targets. Cetuximab is a tyrosine kinase inhibitor (TKI) antibody targeting the receptor for epithelial growth factor (EGFr) on tumor and normal cells. Ipilimumab is an antibody that targets antigen-4 on cytoxic T-lymphocyte (CTLA-4), an antigen that disables cytoxic T-lymphocytes from engaging foreign tumor cells in the body. By this action, ipilimumab augments T-cell activation and proliferation to attack cancer cells. Nivolumab is an antibody targeting PD-1 ligands known to combine with accessory ligands (PD-L1 and PD-L2) to activate the PD-1 receptor on cytotoxic T-lymphocytes. Activation of PD-1 receptor inhibits the proliferation and cytokine production of anti-cancer cytotoxic T-lymphocytes. Thus, defacto blockage of this inhibitory PD-1 receptor by nivolumab, allows cytotoxic T-cells to proliferate, generate cytokines and kill cancer cells. In this case series each agent gave rise to toxic mucositis of the oral and gastrointestinal tract with symptoms that range from pain, difficulty swallowing, nausea, vomiting and diarrhea.
Anti-mucositis therapies
Most FDA approved options: Most FDA approved options for management of toxic mucositis have only fractional effects in the form of moderate pain attenuation with minor effects on the incidence or severity of toxicity. With 75% of patients unresponsive to anti-mucositis therapies [45,46] reduction in the toxicity is insignificant, as are effects on morbidity, costs of care and mucositis-mediated premature mortality. Only complete prevention or rapid reversal could reasonably impact the health economic effects of toxic mucositis.
FDA approved HPPCLS: However in this case series of seven cancer patients treated with ten different anti-neoplastic regimens, the anticipated course of toxic mucositis was averted by the use of high potency polymerized cross-linked sucralfate (HPPCLS). Regular sucralfate suspension, paste or tablets are ineffective on toxic mucositis [47] and except for enema administration for radiation proctitis, the use of sucralfate is not supported by the clinical guidelines of the Multi-national Association of Supportive Cancer Care (MASCC) [48]. The use of HPPCLS in these seven patients resulted in unexpected clinical outcomes. Four patients who developed toxic mucositis from 10 different anti-neoplastic therapies experienced rapid (2-3 day) elimination of mucositis, an effect that was sustained during ongoing cancer treatment with continued administration of HPPCLS. Three patients anticipated to develop Grade 3-4 toxic mucositis developed no mucositis when HPPCLS was used at the commencement of chemoradation, in effect experienced complete prevention.
Distinction between polymerized cross-linked sucralfate and generic sucralfate: There is significant pharmacological difference between the generic sucralfate and PCLS. Three hours following administration the maintains a mucosal concentration of sucralfate that is 7 fold greater on normal mucosal lining and 23 fold greater on ulcerated lining compared to its non-polymerized and non-cross-linked counterpart [49]. The three hour post-administration comparative potency of HPPCLS suspension and generic sucralfate suspension can be depicted in Figure 1 which illustrates the 800% and 2,400% greater surface concentration of sucralfate.
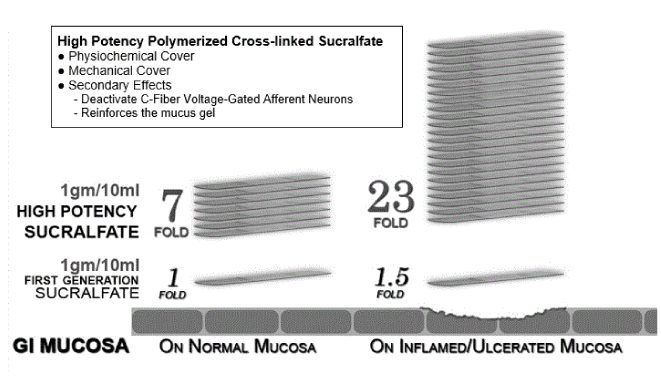
Figure 1. Prolonged exaggerated muco-adherence of polymerized cross-linked sucralfate.
Efficacy and administration of generic sucralfate and of HPPCLS: Meta-analysis of seven randomized controlled trials [46] where 208 patients received 10% sucralfate suspension and 188 patients received either placebo or standard oral hygiene, mucositis was prevent 2% of the patients, grade 2 or higher mucositis was prevented in 25% of patients and grade 3 to 4 mucositis was prevented in 33% of the patients who had otherwise developed mucositis in control patients. Patients in those studies required daily dose of sucralfate ranging from 4,000 to 6,000 mg that was swished, gargled and swallowed or expectorated.
The protocol used in patients of this case series followed the prescribing guidelines shown in Table 4, which are divided according to treatment intent and anticipated severity of mucositis.
Table 4. Single agent protocol using HPPCLS (ProThelial™) for chemo-radiation toxic mucositis
Management Goal &
Anticipated
Severity |
Cancer Therapy
|
Loading Dosing |
Maintenance Dosing
through 1 week
post- cancer therapy |
Treatment Grade 1,2 |
Immuno/Standard chemo-radiation |
2.5-5 ml TID×1 day [250–500mg] |
2.5-5 ml BID [250 – 500mg] |
Treatment Grade 3,4 |
Immuno/Standard chemo-radiation |
10 ml TID×2 days [1000mg] |
5-10 ml BID [500 – 1000mg] |
Prevention Grade 1,2 |
Immuno/Standard chemo-radiation |
2.5-5 ml TID×1 day [250–500mg] |
2.5-5 ml BID [250 – 500mg] |
Prevention Grade 3,4 |
Immuno/Standard chemo-radiation |
10 ml TID ×2 days [1000mg] |
10 ml TID [1000mg] |
Prevention regimen start first day of cancer treatment; BID is twice daily; TID is three times daily
In this case series, patients were instructed to use their tongue to apply dose to all surfaces inside mouth, then gargle for 10 seconds, hold in their mouth for 15 seconds and then expectorate or swallow if so instructed by their clinicians. If tongue application was difficult, then cotton tipped swabs were used to apply HPPCLS onto all oral surfaces, followed by gargling. Patients were assured by clinicians that HPPCLS was safe to swallow, in adults (age 12 and older) 1 gram four times daily for up to 56 continuous days if needed.
Unlike generic sucralfate suspension requiring doses of 4-6 grams daily, daily doses of HPPCLS paste did not exceed 3 grams and in this case series, it was found 100% effective in preventing all grades, especially the anticipated severe grade 3,4 oropharyngeal mucositis. HPPCLS was also observed to be 100% effective in rapid and complete reversal of mucositis occurring in any location within the GI tract caused by any type of cancer treatment regimen. Additionally, while in continuous daily use, HPPCLS-mediated reversal of mucositis was sustained despite ongoing chemo-radiation.
Comparative pharmacodynamics of generic sucralfate and HPPCLS for toxic mucositis: Since both generic sucralfate and HPPCLS are non-systemic in distribution remaining within the GI lumen, pharmacokinetics would not be useful. Pharmacodynamics, that is, molecular handling and mucosal response to generic sucralfate versus HPPCLS would be informative. However, no such reports exist at present. One comparative measure of pharmacodynamics could involve assessment in the change per day in molar concentration of pro-inflammatory cytokines in present in saliva during use of either drug correlated to the patient-reported and clinician-identified clinical effects. This rate of change in the expression of pro-inflammatory cytokines correlated to visible and subjective clinical effects in the mucosa would provide an additional measure of efficacy, one that could be used to assess other anti-mucositis interventions.
Statistical assurance of the veracity of HPPCLS outcomes bring to fore, several implications regarding toxic mucositis, regarding the widely accepted five phase model of mucositis pathobiology. There are implications to future costs of care, patient morbidity and mucositis-associated cancer deaths. Each discussed separately will provide the full scope of the significance of observations in this case series.
Implication regarding chemoradiation toxic mucositis
Despite the varied causes of mucositis or its severity (Grade 2-4) in these patients, the use of HPPCLS was associated with either rapid, sustained elimination or complete prevention of chemo-radiation toxic mucositis. There are at least four implications of these outcomes become apparent.
First, regardless of the cause of mucositis – whether it’s mitotic disruption, sub-cellular dysfunction of organelles or the secondary targeting of T-lymphocytes - the body’s response to injury is, for the most part, immunologically similar if not identical. In other words, mucositis caused by TKI’s, PD-1 inhibitors, or DNA cytotoxics, are of a singular origin or of different origins that are materially indistinguishable in their response to HPPCLS intervention. Toxic mucositis caused by TKI’s, PD-1 inhibitors or DNA cytotoxic agents respond similarly to HPPCLS.
Second, the anatomic location of mucositis does not present unique challenge to management. Toxic mucositis in the oropharyngeal cavity, esophagus, small bowel and colon respond similarly to HPPCLS. Third, regardless of the severity of mucositis grade (grade 2-4), inherent mucosal mechanism(s) tasked to restore homeostasis, do so roughly within the same time frame of 2-3 days. This speaks to pre-existing genomic controlled feedback mechanisms associated with mucosa features that are accessible to HPPCLS that is topically applied and non-systemic.
Fourth, prior to the initiation of mucositis, there must be a operable mechanism(s) inherent to the mucosa, mechanism(s) that could be engaged by an intervention before the onset of toxic mucositis. These inherent mechanism(s) are likely overwhelmed by the onset of pro-inflammatory reaction incited by chemoradiation. Prohibitory or inhibitory system(s) must exist and are tasked, constitutively, with maintaining of mucosal homeostasis. While the nature of these mechanisms or systems are unclear, their existence are verified by the outcomes observed with HPPCLS.
Implications regarding mucositis pathobiology
Complete prevention and rapid sustained elimination of toxic mucositis by any intervention defies the conventional 5 phase model of mucositis [50-53], as the model does not anticipate therapeutic prevention. If toxic mucositis can indeed be prevented and its rapid elimination be sustained in the midst of ongoing chemoradiation, then the 5 phase model does not accurately reflect the pathophysiology of this toxicity. Another model would be required to accommodate the clinical observation of complete prevention and sustained elimination in the setting of ongoing chemoradiation. From these results, obviously the widely recognized five-phase model for mucositis pathobiology [54] cannot account for the clinical outcomes seen with HPPCLS. Complete prevention and rapid reversal of toxic mucositis sustained during ongoing chemoradiation requires a model that can account for these HPPCLS – mediated outcomes.
Implications regarding costs of care, patient morbidity and mortality
Complete prevention and rapid sustained elimination are important clinical outcomes that, if incorporated into oncology practice, would deliver significant health economic benefits. Firstly, prevention and rapid elimination of toxic mucositis would eliminate mucositis-mediated morbidity (pain, difficulty swallowing, dehydration, cachexia, anorexia). Secondly, the elimination of morbidity due to mucositis will reduce the $5.7 billion dollars [20-23] currently spent on 212,785 patients with poorly managed toxic mucositis of the oropharynx and esophagus. Elimination of toxic mucositis permits optimal dosing of chemoradation, similar to the impact of anti-emetics on immediate and delayed onset nausea and vomiting from chemotherapy. Thirdly, optimized dosing of chemoradiation due to mucositis elimination will optimize 5 year survival among patients with toxic mucositis. Lastly, the 11% annual infection-related mucositis deaths in HSCT patients [18,19] will be significantly reduced if not eliminated completely.
The practice of clinical medicine involves seeing one patient at a time, assessing their clinical condition for the application of population-based therapies vetted through randomized controlled trials. Because the vast majority of interventions result in only incremental effects, the control of confounding factors of bias is critical. That criticality is lost when the magnitude of the treatment effect of an intervention outstrips the combined contribution of placebo and selection bias by 10 fold. Such is the case with HPPCLS which has a positive Glasziou treatment effect of 48 to 82, well north of the rate ratio of 10 required to meet statistical significance (p<0.05). Unexpected prevention, complete, rapid and sustained elimination in this series of patients lowered the expected 60-102 days of patient-reported mucositis to 2-3 days and completely prevented it in three patients where prevention was the intent of treatment using HPPCLS. There were several key observations in this case series. It would appear that regardless of the mechanism of action of an anti-neoplastic treatment, the resultant mucositis is indistinguishable in terms of the tissue’s response to injury, differing only in degree of severity of injury. That HPPCLS, a single agent with a single mode of action, can prevent or rapidly reverse toxic mucositis caused by 10 different and distinct anti-neoplastic agents, implies that the molecular nature of toxic mucositis for all 10 agents are the same. Debates as to anti-mucositis therapy options specific to the agent causing mucositis have to accommodate the fact that these 10 agents responded identically to a single anti-mucositis therapy, HPPCLS. Also noteworthy is the tendency toward rapid restoration to homeostasis (2-3 days versus 60-102 patient-reported days [44] regardless of the severity of mucositis. This supports the existence of genomic controlled feedback mechanisms that are active in normal mucosa prior to cancer treatment, and that, prior to HPPCLS, remain active despite morphological worsening of mucositic injury. This notion is new and can be inferred by the magnitude of the treatment effect observed with HPPCLS, namely that homeostasis is either entirely preserved (in the case of prevention by HPPCLS) or rapidly restored (in the case of active mucositis) with restoration sustained by HPPCLS throughout ongoing chemoradiation.
Additionally, to cause complete prevention of mucositis during cancer treatment, some specific (yet to be identified) molecular feature(s) of mucosal homeostasis is (are) engaged/triggered by topically active, non-systemic HPPCLS. These same (yet to be identified) molecular feature(s) tasked with maintaining homeostasis, are also engaged to sustain reversal of mucositis during continued exposure to mucositogenic cancer treatment. These suppositions, supported by the clinical outcomes of HPPCLS, imply that the five phase pathobiology model for mucositis [50-54] is likely incomplete and require modification to accommodate the existence of topically accessible mucosa-associated membrane feature(s) that are tasked with active homeostasis regardless of the grade of CRTM.
Lastly, it is hoped that the outcomes reported here and elsewhere [36,38,39,55] are confirmed by widened use of HPPCLS in the management of chemoradiation toxic mucositis. If so, then there will be significant benefit to cancer treatment patients in terms of reductions to morbidity, to costs of care and to mortality associated with toxic mucositis. Zero tolerance for chemoradiation toxic mucositis may be in the offing.
- Keefe DMK, Brealey J, Goland GJ, Cummins AG (2000) Chemotherapy for cancer causes apoptosis that precedes hypoplasia in crypts of the small intestine in humans. Gut 47: 632–637. [Crossref]
- Al-Sadi R, Ye D, Said HM, Ma T (2010) IL-1beta-induced increase in intestinal epithelial tight junction permeability is mediated by MEKK-1 activation of canonical NK-kB pathway. Am J Path 177: 2310-2322. [Crossref]
2021 Copyright OAT. All rights reserv
- Hebers AHE, deHaan AFJ, vanderVelden WJFM, Donnelly JP (2014) Mucositis not neutropenia determines bacteremia among hematopoietic stem cell transplant recipients. Transpl Inf Dis 16: 279-285. [Crossref]
- Keefe DM, Cummins AG, Dale BM, Kotasek D, Robb TA, et al. (1997) Effect of high-dose chemotherapy on intestinal permeability in humans. Clin Sci (Lond) 92: 385-389. [Crossref]
- Elting LS, Cooksley CD, Chambers MS, Cantor SB, Manzullo E, et al. (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98: 1531-1539. [Crossref]
- Murphy B (2007) Clinical and economic consequences of mucositis induced by chemotherapy and/or radiation therapy. J Support Oncol 5: 13-21. [Crossref]
- Trotti A, Bellm LA, Epstein JB, Frame D, Fuchs HJ, et al. (2003) Mucositis incidence, severity and associated outcomes in patients with head and neck cancer receiving radiotherapy with or without chemotherapy: a systematic literature review. Radiather Oncol 66: 253-262. [Crossref]
- Elting LS, Keefe DM, Sonis ST, Garden AS, Spijkervet FK, et al. (2008) Patient-reported measurements of oral mucositis in head and neck cancer patients treated with radiotherapy with or without chemotherapy: demonstration of increased frequency, severity, resistance to palliation, and impact on quality of life. Cancer 113: 2704-2713. [Crossref]
- Elting LS, Cooksley CD, Chambers MS, Garden AS (2007) Risk, outcomes, and costs of radiation-induced oral mucositis among patients with head-and-neck malignancies. Int J Radiat Oncol Biol Phys 68: 1110-1120. [Crossref]
- Stiff PJ, Emmanouilides C, Bensigner WI, Gentile T, Blazar B, et al. (2006) Palifermin reduces patient-reported mouth and throat soreness and improves patient functioning in the hematopoietic stem-cell transplantation setting. J Clin Oncol 24: 5186-5193. [Crossref]
- Chaudhry HM, Bruce AJ, Wolf RC, Litzow MR, Hogan WJ, et al. (2016) The incidence and severity of oral mucositis among allogeneic hematopoietic stem cell transplantation patients: a systemic review. Biol Blood Marrow Transplant 22: 605-616. [Crossref]
- Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, et al. (2016) SEER Cancer Statistics Review, 1975-2013, National Cancer Institute. Bethesda, MD, based on November 2015 SEER data submission, posted to the SEER web site, April 2016.
- Lewis DR, Chen HS, Cockburn MG, Wu XC, Stroup AM, et al. (2017) Early Estimates of SEER Cancer Incidence, 2014. Cancer 2017. [Crossref]
- Hayes J, Hoverman JR, Brow ME, Dilbeck CD, Verrilli DK, et al. (2015) Cost differential by site of service for cancer patients receiving chemotherapy. Amer J Manag Care 21: e189-e196. [Crossref]
- Kolodziej M, Hoverman JR, Garey JS, Espirito J, Sheth S, et al. (2011) Benchmarks for value in cancer care: an analysis of a large commercial population. J Oncol Pract 7: 301-306. [Crossref]
- Kaiser Commission on Medicaid and the Uninsured. Oct 2015 Fact Sheet;
- Surveillance, Epidemiology, and End Results (SEER) Program. Cancer Facts & Figures 2015.
- Center for International Blood and Marrow Transplant Reasearch.
- Center for International Blood and Marrow Transplant Reasearch (CIBMTR) 2014 REPORT.
- Elting LS, Cooksley C, Chambers M, Cantor SB, Manzullo E, et al. (2003) The burdens of cancer therapy. Clinical and economic outcomes of chemotherapy-induced mucositis. Cancer 98: 1531-1539. [Crossref]
- Nonzee NJ, Dandade NA, Markossian T, Agulnik M, Argiris A, et al. (2008) Evaluating the supportive care costs of severe radiochemotherapy-induced mucositis and pharyngitis. Results from Northwestern University costs of cancer program pilot study with head and neck and Nonsmall cell lung cancer patients who received care at a County Hospital, a Veterans Administration Hospital or a Comprehensive Cancer Care Center. Cancer 113: 1446-1452. [Crossref]
- Sonis ST, Oster G, Fuchs H, Bellm L, Bradford WZ, et al. (2001) Oral mucositis and the clinical and economic outcomes of hematopoietic stem-cell transplantation. J Clin Oncol 19: 2201-2205. [Crossref]
- Elting LS, Shih YC, Stiff PJ, Bensinger W, Cantor SB, et al. (2007) Economic impact of palifermin on the costs of hospitalization for autologous hematopoietic stem-cell transplant: analysis of phase 3 trial results. Biol Blood Marrow Transplant 13: 806-813. [Crossref]
- Craver C, Gayle J, Balu S, Bunchner D (2011) Clinical and economic burden of chemotherapy-induced nausea and vomiting among patients with cancer in a hospital outpatient setting in the United States. J Med Econ 14: 87–98. [Crossref]
- Burke TA, Wisniewski T, Ernst FR (2011) Resource utilization and costs associated with chemotherapy-induced nausea and vomiting (CINV) following highly or moderately emetogenic chemotherapy administered in the US outpatient hospital setting. Support Care Cancer 19: 131-140. [Crossref]
- Dranitsaris G, Maroun J, Shah A (2005) Severe chemotherapy-induced diarrhea in patients with colorectal cancer: a cost of illness analysis. Support Care Cancer 13: 318-324. [Crossref]
- Elting LS, Shih YC (2004) The economic burden of supportive care of cancer patients. Support Care Cancer 12: 219-226. [Crossref]
- Foote M (1998) The Importance of Planned Dose of Chemotherapy on Time: Do We Need to Change Our Clinical Practice? Oncologist 3: 365-368. [Crossref]
- Russo G, Haddad R, Posner M, Machtay M (2008) Radiation treatment breaks and ulcerative mucositis in head and neck cancer. Oncologist 13: 886-898. [Crossref]
- Cairo MS (2000) Dose reductions and delays: limitations of myelosuppressive chemotherapy. Oncology (Williston Park) 14: 21-31. [Crossref]
- Withers HR, Taylor JM, Maciejewski B (1988) The hazard of accelerated tumor clonogen repopulation during radiotherapy. Acta Oncol 27: 131-146. [Crossref]
- Bonadonna G, Valagussa P, Moliterni A, Zambetti M, Brambilla C (1995) Adjuvant cyclophosphamide, metho¬trexate, and fluorouracil in node-positive breast cancer: the results of 20 years of follow-up. N Engl J Med 332: 901–906. [Crossref]
- Tarnawski R, Fowler J, Skladowski K, Swierniak A, Suwiński R, et al. (2002) How fast is repopulation of tumor cells during the treatment gap? Int J Radiat Oncol Biol Phys 54: 229-236. [Crossref]
- Tomiak AT, Videtici GM, Stitt LW (2000) Treatment breaks caused by toxicity from concurrent chemoradia¬tion for limited small cell lung cancer decrease survival and local control. Presented at the 42nd Annual Meeting of the American Society of Therapeutic Radiation Oncology. October 22–26, 2000. Boston, Massachusetts. Abstract 2124.
- Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, et al. (2003) Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys 56: 399-412. [Crossref]
- McCullough RW (2015) Single agent standardized anti-mucositis protocol–now a possibility. A 66 patient multi-institution phase IV post-market surveillance of Prothelial (high potency polymerized cross-linked sucralfate). Brit J Med Med Res 10: 1-17.
- World Health Organization (1979) Handbook for reporting results of cancer treatment. Geneva, Switzerland, pp: 15-22.
- Cox JD, Stetz, J, Pajak, TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31: 1341-1346. [Crossref]
- McCullough RW (2013) High-potency sucralfate prevents and rapidly reverses chemo-radiation mucositis in a patient with stage 4b head and neck cancer. World J Transl Med 2: 13-21.
- McCullough RW (2015) Single agent anti-mucositis protocol for oral and gastrointestinal mucositis and other implications on current concepts regarding chemoradiation induced mucositis and its management from a phase IV post-market surveillance of ProThelial-high potency polymerized cross-linked sucralfate (HPPCLS). Eur J Oncol Pharm 9: 1-11.
- Oxman AD (2004) Grading quality of evidence and strength of recommendations. Grade of recommendation, assessment, development and evaluation (GRADE) working group. BMJ 328: 1490-1494.
- Glasziou P, Chalmers I, Rawlins M, McCulloch P (2007) When are randomised trials unnecessary? Picking signal from noise. BMJ 334: 349-351. [Crossref]
- Howick J, Friedemann C, Tsakok M, Watson R, Tsakok T, et al. (2013) Are treatments more effective than placebos? A systematic review and meta-analysis. PLoS One 8: e62599. [Crossref]
- McCullough RW (2016) Actual duration of patient-reported mucositis: Far longer than 2 to 4 weeks and may be avoidable altogether. Kor J Clin Onc 12: 1-15.
- Donnelly JP, Blijlevens NM, Verhagen CA (2003) Can anything be done about oral mucositis? Ann Oncol 14: 505-507. [Crossref]
- Clarkson JE, Worthington HV, Eden TOB (2007) Interventions for preventing oral mucositis for patients with cancer receiving treatment (Review). Cochrane Database of Systematic Review, Art. No: CD001973.
- Worthington HV, Clarkson JE, Eden TOB (2007) Interventions for preventing oral mucositis for patients with cancer receiving treatment (Review). Cochrane Database of Systematic Review Art. No: CD000978.
- Lalla RV, Bowen J, Barasch A, Elting L, Epstein J, et al. (2014) MASCC/ISOO Clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer 120: 1453-1461. [Crossref]
- Kashimura K, Ozawa K (1999) Sucralfate Preparations. US Patent 5,968,906.
- Sonis ST (1998) Mucositis as a biological process: a new hypothesis for the development of chemotherapy-induced stomatotoxicity. Oral Oncol 34: 39-43. [Crossref]
- Sonis ST (2004) A biological approach to mucositis. J Support Oncol 2: 21-32. [Crossref]
- Sonis S (2010) New thoughts on the initiation of mucositis. Oral Dis 16: 597-600. [Crossref]
- Al-Dasooqi N, Sonis ST, Bowen JM, Bateman E, Blijlevens N, et al. (2013) Emerging evidence on the pathobiology of mucositis. From the Mucositis Study Group of MASCC. Support Care Cancer 21: 3233-3241.
- Russi EG, Raber-Durlacher JE, Sonis ST (2014) Local and systemic pathogenesis and consequences of regimen-induced inflammatory responses in patients with head and neck cancer receiving chemoradiation. Mediators of Inflammation, Article ID 518261, pp: 1-14.
- McCullough RW (2014) ProThelial (Polymerized cross-linked high potency sucralfate): medical device therapy for treatment and prevention of mucositis. European Journal of Research in Medical Sciences 2: 30-58.

