Remineralization is a fundamental mechanism for caries prevention and generally defined as crystal re-growth of hydroxyapatite in incipient caries lesions due to salivary calcium and phosphate. It is assumed, therefore, that the doses of salivary mineral components would be responsible for promotion of remineralization and could be a clinical biomarker to determine remineralization potential of individuals. Although fluoride is well known environmental factor to enhance remineralization, the impact of salivary mineral levels to remineralization has not been demonstrated sufficiently to date.
The objectives of this study were firstly to determine biological distributions of salivary ion levels of calcium and phosphate among adult populations and secondly to examine the impact of salivary calcium contents to enamel remineralization as measured by combined Quantitative Light-induced Fluorescence (QLFTM) and a human saliva model (HSM) as an ex vivo experimental system employing whole saliva as a reaction mixture for mineral resources.
Part 1: Mineral assay of saliva samples
Paraffin-stimulated whole saliva samples were collected up to 5 ml from 603 adults aged 49.1 ± 12.0 ys old (mean ± SD, range: 23-78 years old; 261 males aged 50.5 ± 11.9 years old and 342 females aged 48.0 ± 12.1 years old) after establishment of informed consent. They were instructed not to eat, drink and brush teeth for at least 2 hours before saliva sampling. The collected saliva samples were then centrifuged at 3,000 rpm for 10 min and the supernatants were subjected for chemical assay of calcium and phosphate ions by means of O-Cresolphtalein (OCPC) and phosphomolybdate methods, respectively using a biochemical analyzer (7700 DDP, Hitachi Co. LTD., Japan) equipped with automated sampler.
Part 2: Remineralization assessment by HSM and QLF
Saliva preparation: Immediately after mineral assay in part 1, 10 saliva samples were randomly selected from samples ranging 0.2-0.6 mM calcium and each 1-ml portion were combined to have 10-ml pooled saliva with low calcium content (0.35 mM calcium after pooled). Another 10 samples were employed from saliva samples ranging 0.8-1.2 mM calcium and combined as well to have middle-range calcium content (0.92 mM calcium after pooled).
Separately, 5 healthy adult volunteers aged 23.5 ± 2.3 years old were invited to collect whole saliva by chewing a calcium enriched gum (POS-CAM, Ezaki Glico Co. LTD., Japan) after establishment of informed consent. They chewed 2 tablets of the gum and spat whole saliva for 10 minutes. The collected saliva samples were centrifuged at 3,000 rpm for 10 min, and each 2-ml supernatant were mixed to have pooled saliva containing higher calcium content (3.8 mM calcium after pooled).
Remineralization process: A total of 18 enamel slabs were cut from freshly extracted bovine incisors and the half areas of the abraded labial surfaces were coated by a transparent nail varnish to maintain as untreated references for QLF analysis. The samples were randomly divided into 3 groups (n = 6 per group) and demineralized by immersion in a 0.1 M lactic acid solution (pH4.5 adjusted by 10M KOH, 100 ml per 6 samples) at 37 oC for 48 hours.
Subsequently, the demineralized samples were statically immersed in (A) pooled human whole saliva containing 0.35 mM calcium, (B) pooled whole saliva containing 0.92 mM calcium or (C) pooled whole saliva stimulated by chewing a calcium enriched gum (3.8 mM calcium) for 24 hours at 37oC (n = 6 per group). The saliva volume was 10 ml per 6 enamel samples. Subsequently, fluorescence images were captured by QLF clinical system (Inspektor Pro, Inspektor Research Systems bv, The Netherlands) in darkroom before and after the saliva treatments. Finally, ΔF recovery values (ΔΔF, %) values were assessed by subtracting ΔF after demineralization (ΔFD) from ΔF after saliva treatments (ΔFR; Figure 1).
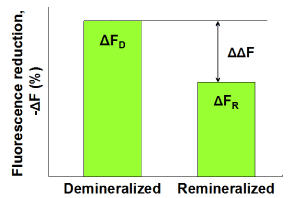
Figure 1. Schematic presentation of ΔF recovery (ΔΔF, %) defined as ΔFR -ΔFD indicating remineralization in enamel lesions.
The calcium ion levels in whole saliva samples ranged extensively from 0.25-2.77 mM (1.12 ± 0.31 mM) and phosphate contents ranged widely from 2.51-12.27 mM (5.05 ± 1.55 mM) as well. There was no statistical difference between male and female groups for both of calcium and phosphate levels.
Typical fluorescence images for the enamel surface in the group B were shown in Figures 2 and 3. It was obvious that fluorescence in the demineralized part recovered by 24-hour treatment with whole saliva containing 0.92 mM calcium.
The ΔΔF values in the groups B (6.3 ± 3.9%) and C (11.4 ± 3.6%) were significantly higher compared to the group A (0.6 ± 0.4%; p<0.001, Tukey-Kramer multiple comparisons), and the group C indicated significantly greater ΔΔF values with respect to the group B (p<0.01; Figure 4).
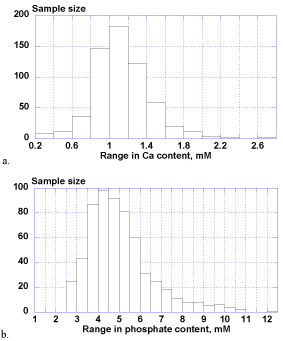
Figure 2. Biological distributions of salivary calcium (a) and phosphate (b).
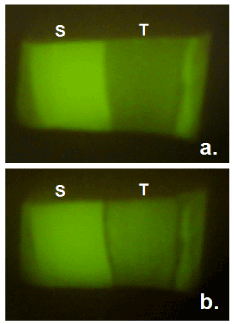
Figure 3. Typical fluorescence images of enamel surface before (a) and after (b) immersion in whole saliva containing 0.92 mM calcium for 24 hours. S = sound reference area protected by nail varnish, T = treated area.
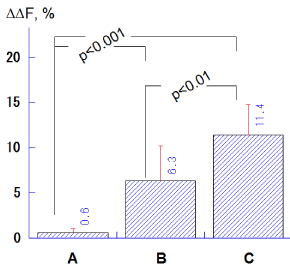
Figure 4. Comparison of ΔΔF (%) values among salivary treatments with different calcium levels (A: 0.35 mM, B: 0.92 mM, C: 3.8 mM). Bar = SD.
With respect to caries formation and prevention, saliva has been studied by especially focusing on mineral saturation status and its relevant factors [1,2]. In contrast, salivary mineral contents were focused in this study and it was found that levels of salivary calcium and phosphate ions ranged extensively among individuals. Such wide distributions would suggest differences in remineralization potential among populations if other relevant factors such as salivary pH, flow rate, water content and mineral saturation status etc are fairly constant.
To examine this hypothesis, demineralized enamel samples were simply exposed to pooled human saliva with different calcium ion levels in part 2. The results revealed 2 significant findings. One is that QLF technology is so sensitive enough to visualize and quantitate mineral changes in saliva induced remineralization within 24 hours. This aspect of QLF is so remarkable if we remind that it takes several weeks or even months of remineralization treatments to obtain measurable remineralization images by radiographic means like transversal microradiography (TMR) [3-5]. As fluorescence reduction parameter ΔF in QLF was known to be well correspondent to mineral loss and lesion depth values by TMR [6,7], its application is surely advantageous in caries research. Another important finding was that remineralization was found to be significantly dependent on calcium ion levels in saliva even supersaturation of mineral is possibly secured. Therefore, it would be reasonable to focus on mineral contents in saliva in addition to its saturation status.
From clinical view point, the present results suggested 2 possibilities. One is that there could be some populations whose remineralization potential is insufficient resulting in at higher risk in dental caries. For such a group at risk, the results of this study revealed one effective solution to recommend calcium enriched agents like a chewing gum containing soluble calcium resources as phosphoryl-oligosaccharide calcium (POs-Ca). From our series of studies on POs-Ca [8-11], it was suggested that the POs-Ca gum can support oral health by optimizing salivary calcium to be suitable for remineralization in enamel and dentin lesions. Another possibility is that the combined methods of the human saliva model (HSM) and QLF could be as a clinical test to screen remineralization (caries recovery) potential of human saliva samples. Such a test to evaluate healthy factor would be highly useful especially to establish a custom-made oral healthcare in addition to caries risk assessment.
Regarding experimental procedures, HSM has limitation that all the salivary factors relating to dental caries cannot be controlled sufficiently. However, if compared to time consuming intra-oral (in situ or in vivo) models, HSM as 'ex vivo' system is advantageous to make experimental procedures simplified and reproducible, especially combined with QLF technology when focusing on remineralization assessment. Also, if compared with chemical models using artificial saliva (mineral solutions) and if remind that one cannot reproduce saliva completely anyway, HSM is highly adapted into the fact that remineralization is a biological phenomenon induced by human saliva itself.
- It was found that concentrations of salivary calcium and phosphate ions vary extensively among individuals suggesting wide variety of remineralization potentials among populations.
- It was found that QLF was sensitive enough to detect saliva-induced mineral recovery of enamel lesions within 24 hours, and the combined methods of the human saliva model (HSM) and QLF could be a clinical test to screen remineralization potential of human saliva samples.
- Gron P (1973) The state of calcium and inorganic orthophosphate in human saliva. Arch Oral Biol 18: 1365-1378. [Crossref]
- Hay DI, Schluckebier SK, Moreno EC (1982) Equilibrium dialysis and ultrafiltration studies of calcium and phosphate binding by human salivary proteins, implications for salivary supersaturation with respect to calcium phosphate salts. Calcif Tissue Int 34: 531-538. [Crossref]
- Arends J, Ruben JL, Inaba D (1997) Major topics in quantitative microradiography of enamel and dentin: R parameter, mineral distribution visualization, and hyper-remineralization. Adv Dent Res 11: 403-414. [Crossref]
- Wang J, Someya Y, Inaba D, Longbottom C, Miyazaki H (2005) Relationship between electrical resistance measurements and microradiographic variables during remineralization of softened enamel lesions. Caries Res 39: 60-64. [Crossref]
- Inaba D, Minami K, Kamasaka H, Yonemitsu M (2002) Remineralization of Enamel and Dentin by A Chewing Gum Containing Phosphoryl-Oligosaccharide Calcium (POs-Ca) in situ. Dent J Iwate Med Univ 27: 203-209.
- van der Veen MH, de Josselin de Jong E (2002) Application of quantitative light-induced fluorescence for assessing early caries lesions. Monogr Oral Sci 17: 144-162. [Crossref]
- Lagerweij M, van der Veen M, Ando M, Lukantsova L, Stookey G (1999) The validity and repeatability of three light-induced fluorescence systems: An in vitro study. Caries Res 33: 220-226. [Crossref]
- Inaba D, Minami K, Kamasaka H, Yonemitsu M (2002) Effects of phosphoryl-oligosaccharides (POs) on remineralization of enamel lesions in vitro. Dent J Iwate Med Univ 27: 197-202.
- Kamasaka H, Inaba D, Minami K, Nishimura T, Kuriki T, Iimai S, et al. (2002) Remineralization of enamel by phosphoryl-oligosaccharides (POs) supplied by chewing gum; Part I. Salivary assessment in vitro. J Dent Hlth 52: 105-111.
- Inaba D, Kamasaka H, Minami K, Nishimura T, Kuriki T, Iimai S, et al. (2002) Remineralization of enamel by phosphoryl-oligosaccharides (POs) supplied by chewing gum; Part II. Intraoral evaluation. J Dent Hlth 52: 112-118.
- Kamasaka H, Inaba D, Minami K, NIshimura T, Kuriki T, Yonemitsu M (2003) Production and application of phosphoryl oligosaccharides prepared from potato starch. Trends Glycosci Glycotechnol 15: 75-89.




