Bone and periodontal infections are associated with inflammation, soft tissue destruction and bone loss. The infection and inflammation are usually managed by administration of oral antibiotics and analgesic drugs while bone fillers are used to repair/regenerate the lost bone. As oral delivery of these drugs suffers from low bioavailability and poor concentration at the infection site, local delivery to infection site may be more beneficial. In this study, we have developed an osteoconductive combinatorial drug delivery system (DDS) composed of monophasic calcium deficient hydroxyapatite (CDHA) Nano carriers aiming at antibacterial, anti-inflammatory and bone regenerative aspects of periodontal infection. Periodontal infection specific antibiotics (tetracycline and doxycycline) were used along with ibuprofen, an anti-inflammatory drug for the current study. CDHA of Ca/P 1.61 (CDHA1.61) which is similar to osteoconductive hydroxyapatite was loaded with antibiotics while CDHA of Ca/P ratio 1.5 (CDHA1.5) of similar composition to resorbable tricalcium phosphate was chosen for loading of ibuprofen. Combined release of these drugs was achieved by formulating a combinatorial CDHA drug delivery system (CDDS) of 50:50 ratio of antibiotic loaded CDHA1.61 and ibuprofen loaded CDHA1.5 nanocarriers. The combined release from CDDS was studied over a period of five days along with individual release. The CDDS showed around (65-75%) antibiotic release and an ibuprofen release of 25 % approx. at the end of 72 h and 24 h respectively. Compared to individual drug release profile from CDHA carriers, CDDS showed a more controlled release of antibiotics and higher ibuprofen release. The CDDS also demonstrated significant in vitro antibacterial activity, anti-inflammatory activity and biocompatibility suggesting possible application of the CDHA based combinatorial DDS for the comprehensive management for periodontitis as well as bone infections.
Periodontitis, calcium deficient hydroxyapatite, antibacterial, anti-inflammatory, local drug delivery, combined release, bone infection
Bone and dental infections such as osteomyelitis, periodontitis etc., are always accompanied by inflammation, tissue destruction and bone loss [1]. Management of bone infections usually involves systemic administration of antibiotics and analgesics. The bone defects created due to bone loss accompanying the infection is locally treated with bone fillers [2]. Since systemic administration of various drugs is associated with side effects and poor concentration at infection site leading to variation in clinical response, it would be ideal to deliver them locally [2]. Local drug delivery systems present multiple advantages such as high concentration of drugs at the target site, prolonged duration of drug action and lower side effects, leading to a more effective and safer treatment [3,4]. Many antibiotics, anti-inflammatory drugs, anti-resorptive drugs, growth factors etc., have been tried for local delivery for bone and dental disorders with a majority of the work focused on the delivery of a single therapeutic agent [5,6]. Current trend is on the delivery of multiple drugs with the reported systems mostly aimed at bone cancer therapy and bone regeneration [7,8]. In case of bone and dental infections, although combinatorial therapies of antimicrobials have been reported [9], drug therapy for the inflammation associated with the infections remains largely unaddressed.
Periodontitis is a complex infectious disease associated with both host and bacteria mediated tissue destruction requiring administration of multiple drugs such as antibiotics, anti-inflammatory drugs etc. [10]. According to the National Health and Nutrition Examination Survey conducted over the period 2009-2012, approximately 46 % of US adults, representing 64.7 million people, had periodontitis with 8.9 % at an advanced stage [11]. Conventional treatment for periodontitis usually involves a) mechanical debridement of periodontal pockets for plaque removal and b) oral administration of antibiotics and anti-inflammatory drugs. Studies have shown that many systemic antibiotics have low concentration in the gingival crevicular fluid (GCF) after oral administration [12,13]. Although bacterial infection is the primary cause of periodontitis, the host-mediated responses triggered by the bacterial enzymes break down the extracellular matrices, leading to subsequent inflammation and bone loss [10]. Therefore, it is more appropriate to develop local delivery systems focused on delivering multiple drugs taking care of all the above aspects namely antimicrobial, anti-inflammatory and bone regeneration for a complete management of periodontitis.
Among various drug carriers, calcium phosphate ceramics (CPC) are ideal candidates for multi-drug delivery to bone due to the presence of multiple functional groups, biocompatibility, biodegradability and compositional similarities to bone mineral [5]. Hydroxyapatite (HA), calcium deficient hydroxyapatite (CDHA) and β-tricalcium phosphate (β-TCP) are some of the CPCs commonly used for biomedical applications [5]. CPCs based local delivery system provides an added advantage of bone regeneration and repair which is desirable for periodontitis treatment. Especially, CDHA, a non-stoichiometric variant of HA with a hexagonal crystal structure has been found to be more suited for such drug delivery applications [14-16].
In the current work, a combined therapy via simultaneous release of both the antibiotics and anti-inflammatory drugs, aiming for an effective treatment of periodontitis using nano CDHAs has been attempted. The tetracycline and doxycycline antibiotics were chosen based on their effectiveness and popular usage for periodontitis. The tetracycline class of drugs is not only antibiotic, but also inhibit osteoclast mediated bone resorption and increase osteoblast activity thereby promoting bone formation [17]. Ibuprofen is a powerful non-steroidal analgesic and anti-inflammatory drug (NSAID) commonly used for pain relief and to reduce inflammation in periodontitis. The local drug delivery system were formulated based on our earlier works where individual release of antibiotics (tetracycline and doxycycline) and anti-inflammatory drug (ibuprofen) was studied from CDHAs of different Ca/P ratios and crystal structures [14-16]. CDHA of Ca/P ratio 1.61 was found to be the best carrier for antibiotics (tetracycline and doxycycline) [14,15] while CDHA of Ca/P ratio 1.5 was found to have the maximum ibuprofen release among the various CDHA systems [16]. Hence, we have developed a CDHA based drug delivery system (CDDS) for the simultaneous delivery of antibiotics and anti-inflammatory drugs as well as to function as a bone regenerative material. The in vitro drug release, antibacterial, anti-inflammatory and biocompatibility activity of the DDS were evaluated for potential clinical applications.
Materials
Analytical grade calcium nitrate [Ca3(NO4)2·4H2O], diammonium hydrogen phosphate [(NH4)2HPO4] and ammonia (30% GR) were purchased from MERCK, India. Tetracycline, doxycycline hyclate and ibuprofen were purchased from Sigma Aldrich, India. Bovine serum albumin (BSA) was purchased from SRL Pvt. Ltd, Mumbai.
Synthesis
CDHA (Ca/P ratio 1.61 and 1.5) nanoparticles were prepared by microwave accelerated wet chemical synthesis method using Ca3(NO4)2·4H2O and (NH4)2HPO4 as precursors solutions [15]. The pH during the synthesis was maintained above 10 using ammonia. After complete mixing, the solution was subjected to irradiation in a microwave oven (BPL, India) of 800 watts for about 30 min at 60 % power. The precipitate was then washed thrice with distilled water to remove ions such as NH4+ and NO32-, oven dried at 100 °C and ground to a fine powder using an agate mortar and pestle. The CDHAs of Ca/P ratio 1.61 and 1.5 were labelled as CDHA1.61 and CDHA1.5 respectively.
Material characterization
The synthesized nanoparticles were analyzed for phase purity by X-ray powder diffraction method (XRD, Bruker D8 DISCOVER, USA) using Cu Kα radiation (λ = 1.54 Å). The diffraction patterns were recorded from scan range of 20-60º with a step size of 0.1º /step and at a scanning rate of 1 step/s. The functional groups present in the nanoparticles were analyzed in the spectral range of 4000–510 cm-1 by Fourier transform infrared spectroscopy (Spectrum Two FT-IR spectrometer, Perkin-Elmer, USA) in the attenuated internal reflection (ATR) mode. Transmission electron microscopy was used to identify the morphology of the nanoparticles. The samples were dispersed in acetone and sonicated using an ultrasonic bath (Citizen, India) at a frequency of 45 kHz for 15 min. The dispersions were dropped on carbon-coated copper grids, dried and examined with a transmission electron microscope (Philips CM20 TEM, Netherlands) operated at 120 kV. Surface area measurements were obtained using Brunauer-Emmett-Teller (BET) isotherm method (Sorptomatic 1990, USA). The samples were degassed at 100º C for 2 h and their surface area was measured at the temperature of liquid nitrogen by the N2 adsorption/desorption isotherm.
In vitro drug loading and release studies
Loading and combined release studies of drugs were performed as described in our previous reports [14-16]. The drugs were dispersed in aqueous/non-aqueous medium depending on their solubility. In case of doxycycline hyclate and ibuprofen, phosphate buffer solution (PBS) of pH 7.4 was used while ethanol was the medium of choice for tetracycline. 1mg of the drugs was dispersed in 10 ml of the medium. 10 mg of CDHA1.61 was added separately to the antibiotic solutions while 10 mg of CDHA1.5 was added separately to ibuprofen solution. The samples were placed in water bath at 37 ºC for 24 h. After 24 h, 2 ml of supernatant was removed for estimation of the drug concentration using UV-Vis spectrophotometer (Lambda 35, Perkin-Elmer, USA). The peak at 274 nm was used for the estimation of tetracycline and doxycycline, while the peak at 222 nm was used for the estimation of ibuprofen concentration. The samples were centrifuged and dried at room temperature for 24 h. The amount of drug-loaded onto the nanocarriers was determined by the following equation:
% Drug loading= [(Ic – Fc)/ Ic] X 100 (1)
Where Ic and Fc are initial and final concentration of the drugs in their respective solvents.
The release study was performed by dispersing drug-loaded nanocarriers in PBS solution of pH 7.4 kept in a constant temperature water bath at 37 °C. Combined release was carried out by dispersing tetracycline/ doxycycline loaded CDHA1.61 nanocarriers and ibuprofen loaded CDHA1.5 nanocarriers mixed at an equal ratio to form the CDDS for two drug combinations, doxycycline:ibuprofen (DI) and tetracycline:ibuprofen (TI). About 2 ml of supernatant was removed for the drug concentration estimation and replaced by fresh PBS at periodic intervals over a period of 5 days. The drug release profile was determined by measuring the absorbance values at different time intervals (Fc) from the initial concentration (Ic). The concentrations of drugs in sample solution were determined using the well-known simultaneous equation method. All the experiments were performed in triplicates.
In vitro antibacterial studies
A drug-loaded CDDS sample (10 mg) was added to 9 ml of the nutrient broth. Pure CDDS samples were taken as control. The suspensions were then inoculated with 1 ml of S.aureus and E.coli bacterial cultures and incubated at 37 °C for 24 h with shaking. The antibacterial efficacy of the drug-loaded samples was determined from the optical density (OD) of the cultures at 600 nm. The antimicrobial reduction percentage was calculated using the following equation:
% Bacterial reduction = [1 – (Sample OD/ control OD)] x 100 (2)
In vitro anti-inflammatory studies
The anti-inflammatory activity of drug-loaded CDDS was studied by the inhibition of albumin denaturation method [18]. 1 % aqueous solution of BSA was prepared and the pH was adjusted to 6.5 with glacial acetic acid. 10 mg of the drug-loaded CDDS samples were suspended in 10 ml PBS and incubated at 37 °C for 24 h. 1 ml of the supernatant from the drug-loaded CDDS samples was added to 3 ml of BSA solution following which it was heated to 51 ºC for 20 min. The solution was then cooled and the turbidity was measured at 660 nm by UV-Visible spectroscopy. The experiment was performed in triplicate with pure CDDS as control. The percentage inhibition of protein denaturation was calculated as follows:
Percentage inhibition = (Ac –As) / Ac X 100 (3)
Where Ac = absorbance of BSA, As = absorbance of BSA-CDDS
In vitro biocompatibility studies
The biocompatibility of the drug-loaded nanoparticulate CDDS was tested against swiss 3T3 fibroblast cells (NCCS, Pune) by MTT [3-(4, 5-181dimethylthiazole-2-yl)-2, 5-diphenyl tetrazolium bromide] assay. MTT assay is a colorimetric test based on the selective ability of viable cells to reduce the tetrazolium component of MTT into purple colored formazan crystals. The swiss 3T3 fibroblast cells were grown to confluence with Dulbecco's modifed eagle's medium (DMEM) supplemented with 10 % fetal bovine serum (FBS) and 1 % 100X antibiotic-antimycotic liquid and incubated at 37 °C with 5% carbon dioxide in a CO2 incubator (Astec, Japan). The cells were then trypsinized and the number of cells was counted with the help of a haemocytometer (Marienfeld, Germany). They were then diluted (104 cells per well) and seeded in 96-well plates and cultured for 24 h. 10 mg of the CDDS samples were suspended in 1 ml of DMEM and incubated at 37 °C for 24 h. The media in the 96-well plates was then replaced with 100 μl of the supernatant from the CDDS samples and again incubated for 24 h. 20 μl of 5 mg/ml MTT was added to each well and incubated for 4 h. The formazan precipitates were solubilized in dimethyl sulfoxide (DMSO) and the absorbance was measured at 570 nm using a multimode plate reader (EnSpire, Perkin-Elmer, Singapore). The percentage of viable cells was calculated as the percentage relative to the control (standard polystyrene tissue culture plates) using the following equation:
% Cell viability = (Sample OD/control OD) x 100 (4)
Statistical analysis
The values are expressed as mean ± SD. Statistical analysis was performed using one and two way ANOVA wherever applicable. The p-value <0.05 was considered statistically significant.
Material characterization
The XRD patterns of the CDHA1.61 and CDHA1.5 nanoparticles along with typical combined drug-loaded CDDS system are shown in Figure 1. The CDHAs were compared with the standard hydroxyapatite pattern (JCPDS 09-432). All the monophasic patterns were found to be similar to their respective standards confirming their purity. The average crystallite size of the nanoparticles (t) was calculated using Scherrer’s formula [t=0.9λ/ B Cosθ] where λ = wavelength of CuKα radiation and B = full width at half-maximum value (in radians) of the diffraction peak (2θ). The diffraction peak at 26° corresponding to (0 0 2) plane of CDHA was chosen for the crystallite size calculation. The cell parameters and cell volume were calculated from the XRD data using the program ‘UnitCell’ and are listed in table, along with their crystallite size. The XRD patterns of the drug-loaded CDDS also shows the presence of CDHA peaks which confirmed that the drug loading procedure did not affect the phase purity of the nano carriers. The TEM images of the as synthesized CDHA Nano carriers are shown in Figure 2. CDHA nanoparticles had platelet like morphology. The particle size of the CDHAs obtained through TEM is given in the table. The BET data listed in the table indicates that CDHA1.5 has a larger surface area of 63 m2/g than CDHA1.61 (41 m2/g).
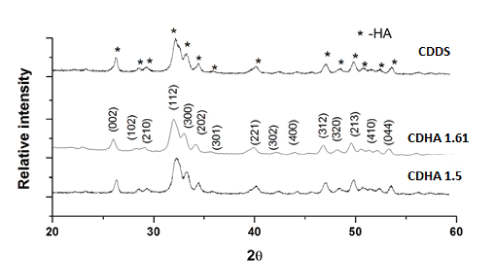
Figure 1: XRD pattern of CDHA nanoparticles along with TI-CDDS sample.
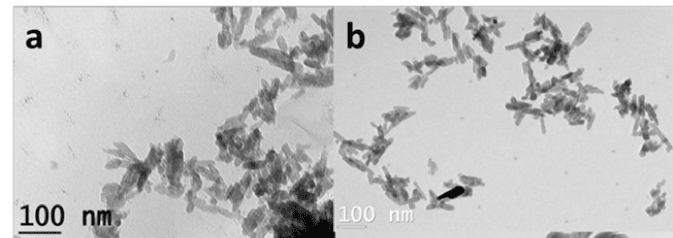
Figure 2: TEM image of a) CDHA1.61 and b) CDHA1.5 nanoparticles.
Table 1: List of cell parameters, particle size and surface area of CaP nanoparticles.
Samples |
Crystal structure |
Cell parameters |
Cell volume
(Å3) |
Particle size (nm) |
Surface area m2/g |
a (Å) |
c (Å) |
XRD |
TEM
(LXW) |
CDHA1.61 |
Hexagonal |
9.389 |
6.639 |
526.87 |
32 |
45X5 |
41 |
CDHA1.5 |
Hexagonal |
9.372 |
6.897 |
524.73 |
32 |
43X6 |
63 |
The functional groups present in CDHA nanoparticles were identified using FT-IR spectroscopy and are shown in Figure 3. All the characteristic vibration bands of CDHA such as PO43− (564, 603, 962 and 1032 cm−1), structural OH− (633 and 3570 cm−1), HPO42- at 876 cm-1 and CO32− (1403 and 1455 cm−1) were present in both CDHA1.61 and CDHA1.5 nanoparticles. The typical FT-IR spectra of the drug-loaded CDDSs are shown in Figure 4. Pure drugs show multiple peaks in the spectral region from 510-1700 cm-1 which was also observed in the drug-loaded CDDSs confirming the presence of drug molecules with the CDHA nanocarriers.
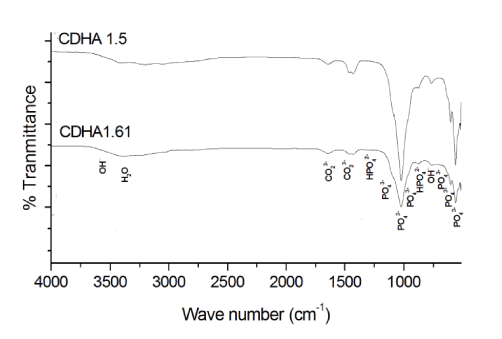
Figure 3: FT-IR spectra of CDHA1.61 and CDHA1.5 nanoparticles.
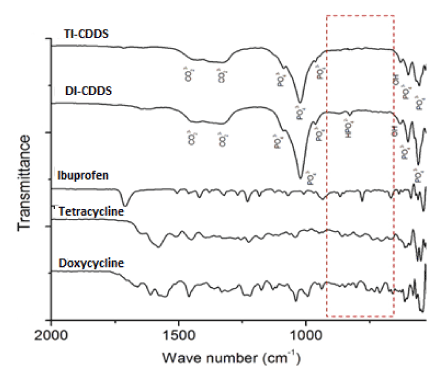
Figure 4: FT-IR spectra of the individual drugs and drug-loaded CDDS samples.
In vitro drug loading and release studies
CDHA1.61 showed a loading of 65±5 % for doxycycline and 70±6 % for tetracycline while CDHA1.5 showed a loading of 75±5 % for ibuprofen at the end of 24 h. The results were similar to our earlier reports [12-14].
The combined release profile of antibiotics and anti-inflammatory drug from the CDDS is shown in Figure 5. In case of TI combinations, CDDS showed the maximum release of tetracycline (75±7 %) and ibuprofen (24±4 %) at the end of 72 h and 24 h respectively. For DI combinations, CDDS showed a doxycycline release of 65±7 % and ibuprofen release of 25±6 % at 72 h and 24 h respectively. In both cases, the CDDS showed a controlled release of antibiotics when compared with their release from CDHA1.61 [14,15]. CDHA1.61 showed nearly 70 % release of antibiotics at the end of 12 h when compared to CDDS. The release was monitored upto five days and an incomplete release was observed which may be attributed to their strong binding with the nanocarriers. The ibuprofen release from CDDS was also higher compared to that from CDHA1.5 [16]. At the end of 24 h, around 20-25 % ibuprofen was released which was nearly twice that of its release from CDHA1.5 (10-12 %).
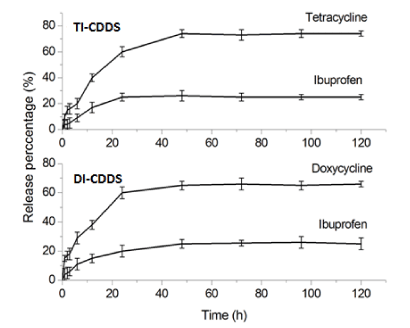
Figure 5: Combined drug release from CDDS for TI and DI combinations.
In vitro antibacterial, anti-inflammatory and biocompatibility studies
The results of antibacterial studies are shown in Figure 6. The in vitro antibacterial activity of the CDDS showed them to be highly active against S.aureus and E.coli. Both TI and DI loaded CDDS nanocarriers show good antibacterial activity between 50–60 % compared to the pure CDDS (control). TI-CDDS was more effective against both the bacterial strains compared to DI-CDDS samples. Since both tetracycline and doxycycline are more effective against Gram- positive bacteria, S.aureus culture showed higher percentage of reduction compared to E.coli. The statistical analysis was calculated by one-way ANOVA with p<0.005.
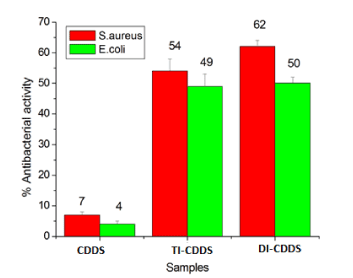
Figure 6: Antibacterial activity of pure and drug-loaded CDDS samples against S. aureus and E. coli.
Figure 7 shows the result of BSA anti-denaturation study to evaluate the anti-inflammatory activity of TI-CDDS and DI-CDDS samples. It can be seen that the BSA denaturation was inhibited by 34±3 % with TI-CDDS samples and 32±4 % with DI-CDDS samples confirming that the anti-inflammatory activity was retained in these samples. Statistical analysis was conducted by one-way ANOVA test with p<0.005.
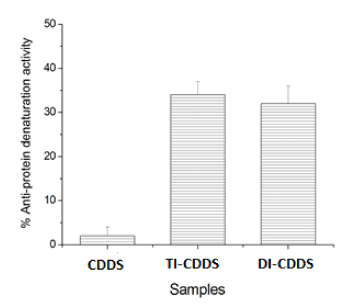
Figure 7: Anti-protein denaturation activity of pure and drug-loaded CDDS samples.
2021 Copyright OAT. All rights reserv
The results of the MTT assay on Swiss 3T3 fibroblast cells performed at 1 mg/ml nanocarrier concentration for 24 and 48 h are shown in Figure 8. Pure CDDS was used as control. All drug-loaded samples were found to be biocompatible and all the samples showed higher cell viability at 24 h compared to the 48 h samples. The statistical analysis was conducted by two-way ANOVA test with a significance of p<0.005.
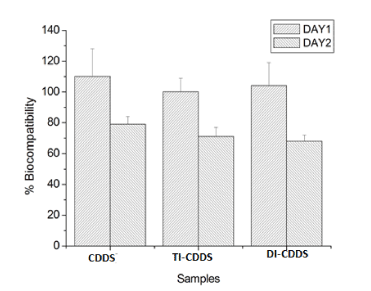
Figure 8: Biocompatibility of pure and drug-loaded CDDS samples evaluated by the MTT assay.
Drug delivery systems capable of delivering multiple drugs provide a comprehensive management by dealing with various aspects of bone infections. CDDS offers a versatile, scalable platform for delivery of multiple drugs coupled with an added advantage of bone repair/regeneration. Our earlier studies have confirmed the potential of CaP nanocarriers as customized drug carriers by tailoring various physico-chemical parameters. These results have prompted further studies on evaluating the capability of these nanocarriers for the delivery of the unique combination of antibiotics and anti-inflammatory drugs. The results of the in vitro antibacterial, anti-inflammatory and biocompatibility studies have confirmed the suitability of CDDS for the combinatorial delivery of antibiotics and anti-inflammatory drugs.
The in vitro analysis of a biocompatible, bioactive nanobioceramic drug delivery system based on CDHAs for combined release of antibiotics and anti-inflammatory drugs aimed at comprehensive treatment of periodontitis shows that the CDHA1.61:CDHA1.5 system can exhibit a co-delivery of antibiotics and ibuprofen. The CDHA system also shows significant in vitro antibacterial and anti-inflammatory activities and therefore can be used for local multi-drug therapy for bone infections.
The authors are grateful to the Department of Biotechnology (DBT), Government of India for funding this work.
Compliance with ethical standards
Not applicable
The authors declare that there is no conflict of interest of a scientific or commercial nature. The authors have no relevant affiliations to, or financial support from any organization that may have a financial interest in the subject matter.
- Bejon P, Robinson E (2013) Bone and joint infection. Medicine 41: 719-722.
- Zilberman M, Elsner JJ (2008) Antibiotic-eluting medical devices for various applications. J Controlled Rel. 130: 202–215. [Crossref]
- Wu P, Grainger DW (2006) Drug/device combinations for local drug therapies and infection prophylaxis. Biomaterials 27: 2450–2467. [Crossref]
- Manzano M, Regi MV (2012) Revisiting bioceramics: Bone regenerative and local drug delivery systems. Prog Solid State Chem. 40: 17-30
- Sampath Kumar TS, Madhumathi K (2016) Antibiotic delivery by nanobioceramics. Ther Deliv. 7:573-588. [Crossref]
- Nguyen S, Hiorth M (2015) Advanced drug delivery systems for local treatment of the oral cavity. Ther Deliv. 6: 197-210. [Crossref]
- D’Arrigo G, Navarro G, Meo CD, Matricardi P, Torchilin V (2014) Gellan gum nanohydrogel containing anti-inflammatory and anti-cancer drugs: a multi-drug delivery system for a combination therapy in cancer treatment. Eur J Pharm Biopharm. 87: 208–216. [Crossref]
- Wang Z, Chui W-K, Ho PC (2011) Nanoparticulate delivery system targeted to tumor neovasculature for combined anticancer and antiangiogenesis therapy. Pharm Res. 28: 585–596.
- Arias PP, Tafin UF, Betrisey B, Vogt S, Trampuz A, Borens O (2015) Activity of bone cement loaded with daptomycin alone or in combination with gentamicin or PEG600 against Staphylococcus epidermidis biofilms. Injury. 46: 249-253.
- Newman MG, Takei H, Klokkevold PR, Carranza FA (2015) Carranza's clinical periodontology. Amsterdam (The Netherlands): Elsevier Ltd.
- Eke PI, Dye BA, Wei L, Slade GD, Thornton-Evans GO et al. (2015) Update on prevalence of periodontitis in adults in the United States: NHANES 2009 to 2012. J Periodontol. 86: 611-622.
- Sakellari D, Goodson JK, Socransky SS, Kolokotronis A, Konstantinidis A (2000) Concentration of 3 tetracyclines in plasma, gingival crevicular fluid and saliva. J Clin Periodontol. 27: 53–60. [Crossref]
- Stoller NH, Johnson LR, Trapnell S, Harrold CQ, Garrett S (1998) The pharmacokinetic profile of a biodegradable controlled-release delivery system containing doxycycline compared to systemically delivered doxycycline in gingival crevicular fluid, saliva, and serum. J Periodontol. 69: 1085-1091.
- Victor SP, Sampath Kumar TS (2008) Tailoring calcium-deficient hydroxyapatite nanocarriers for enhanced release of antibiotics. J Biomed Nanotech. 4: 1-7.
- Madhumathi K, Sampath Kumar TS (2014) Regenerative potential and anti-bacterial activity of tetracycline loaded apatitic nanocarriers for the treatment of periodontitis. Biomed Mater 9: 035002.
- Madhumathi K, Sampath Kumar TS (2013) Effect of structure and composition on ibuprofen drug delivery by calcium phosphate nanocarriers. Key Eng Mater. 529-530: 495-500.
- Payne JB, Golub LM (2011) Using tetracyclines to treat osteoporotic/osteopenic bone loss: from the basic science laboratory to the clinic. Pharm Res. 6: 3121-3129.
- Mizushima Y, Kobayashi M (1968) Interaction of anti-inflammatory drugs with serum proteins, especially with some biologically active proteins. J Pharm Pharmacol. 20: 169-173.








