Abstract
Background
Nucleoside Diphosphate Kinase (NDPK), described as NM23 a metastasis suppressor, is found in the culture medium of cancer cells lines suggesting that the kinase may have an extracellular role. We propose that extracellular NM23 released from breast cancers in vivo stimulates tumor cell migration, proliferation and endothelial cell angiogenesis in support of metastasis development.
Methods
NM23 in the bloodstream of immunocompromised mice carrying human triple-negative breast cancers or in breast cancer patients was measured by ELISA. Primary and metastatic tumor development, the impact of blockade of NM23 and/or its stimulation of nucleotide receptors were measured using in vivo imaging. NM23 expression data in the Curtis breast dataset was examined to test our hypothesis that NM23 may play a mechanistic role in breast cancer development.
Results
SCID mice carrying metastatic MDA-MB-231Luc+ triple-negative human breast tumor cells elaborate NM23 into the circulation correlated with primary tumor growth. Treatment of mice with the NM23 inhibitor ellagic acid (EA) or the purinergic receptor antagonist MRS2179 slowed primary tumor growth. At 16 weeks following implantation, lung metastases were reduced in mice treated with EA, MRS2179 or the combination. Expression of NM23 in the Curtis breast dataset confirmed a likely role for NM23 in tumor metastasis.
Conclusions
Extracellular NM23 may constitute both a biomarker and a therapeutic target in the management of breast cancer.
Key words
breast cancer, NDPK, NM23, P2Y1 receptor, SCID mice, tumor growth, metastasis
Introduction
Breast cancer cells migrate to distant sites in the body before they are capable of forming aggressive metastases and thus can remain dormant [1]. Excision of a primary tumor can cause tumor metastases to appear shortly thereafter [2,3]. Assuming that cells aren’t ‘dislodged’ during surgery, an intellectually displeasing notion, it is reasonable to take this as evidence of distant metastases extant and indolent at the time of adjuvant treatment, and changes in these cells from quiescent to active growth occurring at some future time, perhaps years following surgery. We do not know the precise cellular behaviors underlying disease spread, whether via lymph or blood, but intravasation, extravasation and angiogenesis are early events that precede and are required for the formation of metastatic lesions that become malignant.
Breast cancer specific mortality is almost exclusively a function of metastasis [4]. If cells did not spread, mastectomy, if not lumpectomy might cure breast cancer. Growth of tumor cells as metastases dictates that tumor cells must first enter the blood stream which they do in large numbers [5] and exit into tissue spaces such as the lung and do so having survived multiple potential fates [6]. Thus, these are rare events. What determines the ability of cells to spread to distant sites is said to be their de-differentiation as a result of epithelial to mesenchymal transition (EMT) [7,8], a process promoted by transforming growth factor b [9].
The product of the NME1 and NME2 genes (aka., NDPK-A and NDPK-B) function as nucleoside diphosphate kinases regenerating ATP levels by covalently transferring the g-phosphate from a nucleoside triphosphate (NTP) such as ATP or GTP, to a nucleoside diphosphate acceptor (NDP; e.g., ADP). While this could be seen as a futile conversion when the phosphoryl donor is ATP, the result serves to maintain ADP levels in the environment of the enzyme. In the blood stream, this can mean that nucleotide concentration is maintained in time and space [10]. This is the only known function of circulating NM23, while intracellular NM23 isozymes have been shown to act as a histidine kinase [11], a transcription activator and an exonuclease [12]. Extracellular actions of NM23 play a role in cellular guidance during development [13]. The original description of intracellular NM23 as a metastasis suppressor [14,15] has received considerable attention, but exceptions to this notion have appeared from our lab and others [16-21]. Our data suggest that in breast cancer, only a metastasis suppressor role for Nm23 may not be the case and further, that the extracellular actions of NM23 are distinct from those of overall cellular expression and the intracellular signaling that has been the focus of others [22]. When elaborated into the bloodstream by breast tumor cells, the NDPKinase activity of NM23 serves to maintain the ADP levels that otherwise would be rapidly converted to adenosine.
Although a role for extracellular NM23 in the promotion of metastasis is controversial, the ability of both cancer cell conditioned media and purified NDPK to promote angiogenesis in vitro is well established [19,21]. The elaboration of NM23 by numerous breast cancer cell lines, but not their normal counterparts has been established [21]. The extracellular actions of NM23 likely involve vascular endothelial cell growth factor (VEGF) signaling. VEGF is established as an EMT-associated factor [7]. NM23 can transactivate the VEGF receptor (VEGFR) in the absence of VEGF [21], suggesting a role for NM23 in EMT. Moreover, NM23 binds to and activates the cell surface receptor MUC1 [23] influencing stem cell growth and promoting transendothelial migration consistent with intravasation and extravasation [24]. Therefore, viewing NM23 only as a metastasis suppressor [25] is inconsistent with significant evidence of its extracellular actions. It is also clear that viewing NM23 only as an angiogenic factor when elaborated outside cells also provides an incomplete picture [26]. Using both mammalian and non-mammalian models of angiogenesis, Wieland’s group have suggested that NM23 (NDPK-B) is required for VEGF-induced angiogenesis and contributes to the localization of VEGF receptor type 2 and VE-cadherin at the endothelial adherens junction. These authors interpret their findings as intracellular rather than extracellular actions of NDPK-B in normal versus pathological models. We suspect that these data are indicative of normal processes that are aberrantly regulated in disease.
In order to further our understanding of the role of NM23 in breast tumor development, we have employed the immunocompromised SCID mouse carrying orthotopic human breast cancers. The MDA-MB-231 human triple-negative tumor cells were engineered to express luciferase allowing longitudinal assessment of tumor growth and metastasis in the mouse. If extracellular Nm23 plays a role in angiogenesis development locally and if NM23 appears in the blood stream, then blocking the kinase action of extracellular NM23 or preventing the action of ATP/ADP at the endothelial P2Y1 receptor would affect the ability of MDA-MB-231Luc+ cells to form tumors and metastasize in the mouse.
Materials and methods
Mouse models of human breast carcinoma
MDA-MB-231 luciferase 2 (Luc+) expressing human cancer cells (Caliper Sciences, Hopkinton, MA) were injected into the female SCID mouse mammary fat pad to establish primary tumors. The luciferase-tagged sub-line of MDA-MB-231S cells designated (231-Luc+) permitted imaging of tumors and tracking of metastases. MDA-MB-231Luc+ cells were cultured in Eagle’s Minimum Essential Medium (MEM) containing 10% FBS at 37°C in 5% CO2. Cells were harvested using trypsin (0.05%), washed twice with cold sterile PBS without calcium chloride and magnesium chloride (sigma, MO) and 2.5×106 cells were mixed 50:50 (v/v) with Matrigel (Sigma, MO) and injected s.c into the mammary fat of 4- to 6-week-old female CB-17 SCID mice. Experiments were approved by the Institutional Animal Care and Use Committee of the University of Nevada, Reno.
Blood collection
At two week intervals, the mouse jugular vein was lanced and 100–125 μl of blood collected. Women’s serum samples were obtained from the National Disease Research Interchange (NDRI), or locally under IRB-approved informed consent. Blood was clotted in a serum separator tube (Becton Dickinson, USA) at room temperature for 1–2 hr. Serum was separated by centrifugation at 6,000×g for 10 min and used immediately in experiments or stored at −80°C for later use.
Indirect enzyme-linked immunosorbent assay (ELISA)
Secreted-human NM23 was detected by ELISA using an NM23 isozyme non-specific antibody that recognizes human NM23. Ninety-six well plates (Corning, NY) were coated with recombinant NM23 protein as standard sample (Abnova, Taiwan), or serum test proteins (experimental). Standard Nm23 samples were employed over a range from 0.03 to 100 ng/mL in phosphate buffered saline (containing 0.05% sodium azide); 50 µl aliquots were added to wells and the plate incubated for 2 hr at room temperature (RT) and then overnight at 4°C on a slow shaker. Blocking buffer (100 µl) containing 5% BSA and Tween-20 (0.05%) in phosphate-buffered saline (PBST 0.05%) was added and incubated for 1.5 hr at RT. Plates were then washed once with 150 µl of PBST, incubated with primary mouse anti-NM23 antibody (Abcam, Cambridge, MA USA) diluted with PBST buffer containing 1 mM EDTA and 0.25% BSA (100 µl) for 2 hr at 37°C with slow rocking, and then washed 3 times with 150 µl PBST. Plates were incubated with rabbit anti-mouse IgG horseradish peroxidase (HRP)-conjugate (Southern Biotech, Birmingham, AL) for 2 hr at 37°C with slow rocking and washed 3 times with PBST. Plates were developed by addition of 100 µl of o-phenylenediamine dihydrochloride substrate, (Sigma St. Louis, MO) and absorbance measured at 490 nm using a microplate reader.
Measurement of NM23 in patient serum
We obtained de-identified serum samples from cancer patients (NDRI, Philadelphia, PA, USA) and control samples (no history of cancer or chronic disease) were obtained from UNEVX Labs (Dr. V. Lombardi) who have collected samples of serum from female patients diagnosed with myalgic encephalomyelitis. These samples were used to assess the possibility that NM23 appears in the sera of breast cancer patients as a consequence of their particular disease. Patient NM23 was measured by ELISA as described above.
Detection of primary tumor formation
Mice were injected s.c in the neck with the luciferase substrate d-luciferin. Mice were anesthetized 20 min later under an IACUC-approved protocol followed by tumor imaging for 20 sec using a Caliper Lumina II IVIS instrument (Perkin Elmer, Waltham, MA). Imaging was repeated every other week to monitor tumor growth. The mean tumor volume and mean tumor bioluminescence were measured independently to define the timelines of primary tumor development by using both caliper measurement and IVIS spectral data. The tumor volume sizes by caliper were calculated by the formula tumor volume (mm3)=[length (mm)] x [width (mm)]2 x 0.52. Mice were sacrificed when the primary tumor reached ~2,000 mm3 in volume (~16 wk). Imaging of deep tissue metastases requires animal repositioning (axillary lymph nodes) and was not as reliable for early lung metastatic time points as removing the organ and imaging directly. At the end of the experiment, animals were euthanized and selected tissues were analyzed by ex vivo imaging and processed for subsequent histology.
Immunoblotting of human secreted NM23
To detect human secreted NM23 from mice carrying human tumors, serum samples were concentrated for 30 min at 4˚C and centrifuged at 2000xg using AmiconÒ Ultra-0.5 10 kDa centrifugal filters (Millipore, Bedford, MA). Concentrated samples were analyzed for secreted NM23 protein by immune blot. Proteins were resolved on SDS-PAGE gels, transferred to nitrocellulose membranes and incubated at 4˚C overnight with anti-NM23 mouse mAB (Abcam, Cambridge, MA). Membranes were incubated with secondary antibodies conjugated to Alexa Fluor 680 (Invitrogen, Carlsbad, CA) in 1:1 Odyssey blocking buffer (Licor Biosciences, Lincoln, NE) and PBS with 0.1% Tween-20 (v/v). Bands were detected using the Odyssey Infrared Imaging System (V2.04). In order to determine if secretion of NM23 into the murine circulation by the MDA-MB-231Luc+ tumor cell was particular to this cell type or was a more general phenomenon, we sampled serum from animals carrying the Mary-X tumor representative of inflammatory breast cancer [27] as well as the primary lung adenocarcinoma cell type [28].
RT-PCR
To determine the mRNA level of intracellular human NM23 in primary tumors, total RNA was extracted using a Mag MAX-96 Total RNA Isolation kit (Ambio, Foster City, CA) and reverse-transcribed using SuperscriptII (Invitrogen, Carlsbad, CA) with random hexamer primers (Invitrogen, Carlsbad, CA). Specific Primers [5’- ACC TTC ATC GCC ATC A -3’(sense) and 5’- AAT CCT GTT GCC TCT AA -3’(antisense) ] for hNDPK-B were used in PCR with Go Tag polymerase(Promega, Madison, WI). β-actin was used as a positive control. Standard end point reverse transcription-polymerase chain reaction (RT-PCR) was performed and analyzed with FluoroChem 5500 (Innotech®) imaging system and software.
Detection of metastases by ex-vivo bioluminescence
Lung tissues were harvested at necropsy (16 wk) and bioluminescence was detected with ex-vivo imaging. Briefly, lung tissues were placed individually into wells of a 24-well plate. Luciferin (300 μg/ml) in Krebs buffer was added to cover the tissues, which were then imaged at 20, 30 and 40 seconds using the Lumina II IVIS instrument (level B/FOV 15 cm).
Treatment with MRS2179 and Ellagic Acid (EA)
Mice bearing MDA-MB-231-Luc+ tumors of 100-200 mm3 were randomized into 5 groups of 6 animals per group (group 1, untreated; group 2, MRS2179 by mini-osmotic pump; group 3, ellagic acid in drinking water; group 4, EA and MRS2179; and group 5, endostatin by mini-osmotic pump). We implanted mini-osmotic pumps (ALZETä Technical Services, Cupertino, CA) to administer MRS2179 (200 μM/day) to groups 2 and 4. Pumps contained 100 μl and continuously delivered drugs at the rate of 0.11 μl/hr for 30 days. On day 28, all pumps were removed under sterile conditions and replaced with fresh pumps. Pumps were replaced at 28-day intervals until control tumors reached terminal size (2,000 mm3 in our design, ~16 wk). EA and MRS2179 compounds were first tested for toxicity in 4 mice treated for 2 months beginning with 30, 60 and 120 μg/ml in drinking water (5 ± 0.7 ml/day per mouse) and daily intraperitoneal injection of 100 ul of MRS2179 AT 100, 150, 200 μM. No obvious toxicity or abnormality was observed (data not shown). Tumor growth inhibition (TGI) was determined by comparing the reduction in the mean tumor volume (mm3) and mean tumor bioluminescence between control and treated mice at both the primary and metastatic sites. Tumor metastasis inhibition (TMI) was determined by direct bioluminescence detection of lungs at necropsy.
Gene expression analyses
The publicly available Oncomine database (www.oncomine.org) was used to assess NM23 mRNA expression in Curtis breast dataset 11 of breast tumor subtypes and normal tissues [29]. Breast cancer tumors were sorted by cancer type and the log2 expression of NM23 was compared. The prognostic value of Affymetrix ID: 201268_at in breast cancer was assessed with Kaplan-Meier plots (www.kmplot.com). Samples were segregated by median NM23 expression. Normalized gene expression levels across all samples were gathered for that gene and the median computed (the half-way mark after ordering numerically). Patients whose expression was above the median were termed "high", the patients with expression below the median are termed "low". The cohorts were of equal size.
Statistical analyses
Statistical analyses were performed using InStat Statistical Software (V3.0; GraphPad Software, Seattle, WA). All experiments were tested for normalcy and statistical significance using ANOVA and a p ≤ 0.05 was considered significant. Data points and error bars represent means ± S.E.M. Significance for the Kaplan-Meier plots were generated by km plot [30].
Results
Appearance of human breast cancer cell-secreted NM23 in mice
We have shown that metastatic human breast carcinoma cells secrete/shed NM23 into their surrounding environment when cultured in vitro and this extracellular NM23 acts as a trans-phosphorylase [21]. Furthermore, we demonstrated that extracellular NM23 promotes endothelial cell migration, proliferation [21] and tubule formation in vitro [18,19]. Cells elaborate NM23 as a phosphoprotein into the extracellular environment where the enzyme is able to transfer a phosphoryl group to a nucleoside diphosphate acceptor via a high-energy phosphohistidine intermediate [31,32]. Our underlying hypothesis is that, in immunocompromised mice bearing orthotopic human breast tumors, the tumor cells will elaborate NM23. Secreted NM23 serves to maintain the ATP/ADP levels that favor vasodilation and promote the formation of capillary vessels permissive to intravasation, extravasation, and angiogenesis in support of primary tumor growth and metastasis by mimic of normal mechanisms of diapedesis [33].
Mouse blood collected prior to tumor cell placement and at 2-wk intervals thereafter until week 16 contained increasing levels of NM23 (Figure 1a). Bioluminescent data correlated with manual tumor volume measurement (r2=0.92) as expected (Figure 1b). The relationship of tumor bioluminescence to extracellular appearance of NM23 showed a positive correlation of r2=0.85 (Figure 1c) suggesting that the presence of human NM23 in serum during tumor development is consistent with an action NM23 to stimulate tumor growth. These results are also consistent with the hypothesis that increased tumor growth results in increased NM23 secretion resulting in a potential positive feedback loop.

Figure 1. Detection of secreted NM23 in SCID mice carrying MDA-MB-231Luc+ tumors. Mouse blood was collected prior to tumor cell placement and at 2-week intervals thereafter until week 16. Control animals (square symbols) received injections of diluent only (n=4 mice). An ELISA assay was performed to quantify the amount of secreted NM23 in serum (n=24 mice). (a) In mice carrying breast cancer carcinoma cells, serum NM23 was measured from the first time point (~400 ng/ml) until week 16 (~924 ng/ml). (b) Primary tumor growth was measured by mean tumor volume, and bioluminescence using non-invasive in vivo imaging (Caliper, Lumina II®) versus time (every other week for 16 weeks). (c) The correlation between mean tumor volume and mean bioluminescence is r2=0.92.
We confirmed the expression of secreted NM23 in the serum of mice bearing human tumors by Western blot analysis (Figure 2a). We found expression of both isozymes of extracellular NM23(~17 and 19 kDa) in samples from nude mice (nu/nu) carrying xenografts of human triple negative inflammatory breast cancer (Mary-X) and bronchio alveolar carcinoma (BAC) as well as in samples from SCID mice carrying MDA-MB-231Luc+ tumors (Figure 2a lane 4-6). NM23 was not detected in concentrated serum from control mice. Importantly, we detected NM23 in the serum of women with breast cancer (Figure 2, lane P1-4), however there was no detectable expression of extracellular NM23 in healthy women (lane V1,2).
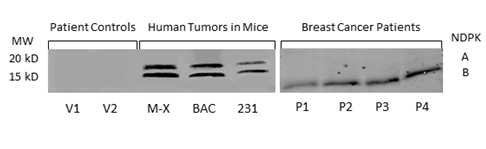
Figure 2. NM23 in the serum of healthy women, breast cancer patients and mouse models of human cancer. NM23 was detected by Western blot in patient controls (V1-2), mice carrying the inflammatory Mary X breast cancer (M-X), bronchioloalveolar carcinoma (BAC) and the human triple negative MDA-MB-231 tumor (231). NM23 was also measured in the serum of breast cancer patients with metastatic disease (P1-4). Blots are representative of multiple experiments.
These data are consistent with our previous observation that NM23 is not secreted by non-tumorigenic MCF-12F mammary epithelial cells [21]. Measurement of NM23 mRNA expression in the tumors (Figure 3a) and NM23 levels (Figure 3b) in the serum of animals carrying the Mary-X and BAC tumors sampled at 16 wk, showed that these tumors expressed high levels of NM23 messenger RNA levels and were secreting NM23.
Because intracellular NM23 expression has been used to define NM23 as a metastasis suppressor, we explored whether NM23 mRNA expression is upregulated in breast cancer patients when compared to patients with no evidence of breast cancer. NM23 expression was examined in the Curtis breast dataset using the publicly available Breast Cancer Gene-Expression Miner [29]. The data revealed that NM23 mRNA expression is upregulated in breast tumor patients when compared to healthy women (Figure 4a, 4b).
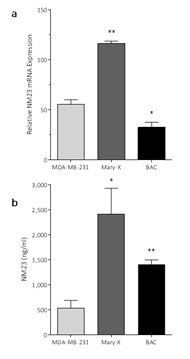
Figure 3. Detection of NM23 and NM23 mRNA expression in mice carrying xenotransplant tumors
- NM23 mRNA expression in primary tumors and in (b) serum from mice carrying MDA-MB-231, inflammatory Mary-X or bronchioloalveolar carcinoma (BAC). Data are mean ± SEM, n=4-6. *=p<0.05, **=p<0.01.
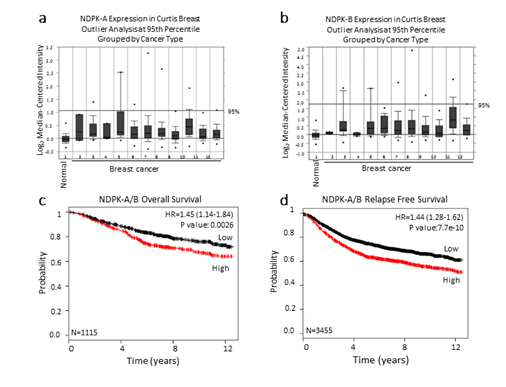
Figure 4. NM23 expression in the Curtis breast data set
The publicly available database Oncomine was used to assess (a) NDPK-A and (b) NDPK-B mRNA expression in the Curtis breast dataset of (1,992) breast carcinoma samples and (144) normal breast samples. The microarray of breast genomic and transcriptomic architecture datasets was accessed using 1=normal breast (n=144), 2=benign breast neoplasm (n=3), 3=breast carcinoma (n=14), 4 =breast phyllodes tumor (n=5), 5=ductal breast carcinoma in situ (n=10), 6=invasive breast carcinoma (n=21), 7=invasive ductal and invasive lobular breast carcinoma (n=90), 8=invasive ductal breast carcinoma (n=1,556), 9=invasive lobular breast carcinoma (n=148), 10=medullary breast carcinoma (n=32), 11=mucinous breast carcinoma (n=46), 12 =tubular breast carcinoma (n=67) and n=sample number. The log2 median-centered ratios for NDPK-A/B (NM23) expression level are depicted in box and whisker plots. Dots represent maximum and minimum outliers. Datasets were sorted by outlier analysis at the 95th percentile and grouped by cancer type. (c, d) Kaplan-Meier survival curves of breast cancer patients using publicly available clinical breast cancer database. Breast cancer patients were grouped to high and low expression of NDPK-A/B. NM23 levels with a median follow-up period of 12 years. The plots display the probability of patients overall survival (c), and relapse free survival (d). Log-Rank test was used to analyze survival differences.*P<0.01 vs control.
Increased NM23 expression correlates with decreased over all and relapse-free survival
We further examined the correlation of median NM23 (NDPK-A/B) mRNA expression levels with overall survival and relapse-free survival using a Kaplan-Meier plot [30]. Importantly, breast cancer patients with low levels of NM23expression displayed significantly prolonged overall survival and relapse-free survival rates compared to patients having relatively higher levels of NM23 (Figure 3c, 3d). Together, these results fit with our hypothesis that NM23 is a critical regulator of metastasis in human breast cancer and extracellular nucleotide kinase correlates with poor prognosis in breast cancer patients.
We reasoned that if NM23 is elaborated from breast cancer cells implanted in mice and appears in the serum, and is seen on Western blots of the serum from patients with ductal carcinoma of the breast (Figure 2), then it can be measured by ELISA in the sera from patients with breast cancer. We obtained de-identified serum samples from cancer patients (NDRI) and control samples (no history of cancer or chronic disease) from UNEVX Labs (Dr. V. Lombardi) who have collected samples of serum from female patients diagnosed with ME/CFS. These samples are the first step in assessing the possibility that NM23 appears in the sera of breast cancer patients as a consequence of their particular disease (Table 1). Our data demonstrate that NM23 circulates in the bloodstream of female patients with breast cancer metastases, but not a gender-controlled population with unrelated chronic disease (ME/CFS) or patients without known disease (control). Moreover, low levels of NM23 are seen in patients with ductal carcinoma in situ (Table 1).
Patients |
Number of Subjects |
NM23 (ng/mL Serum) |
Age (mean ± SEM) |
Diagnosis |
10 |
0.02 ± 0.01 |
32 ± 3 |
Control |
4 |
54.2 ± 22.3 |
41.75 ± 14 |
Bone metastases |
9 |
74.28 ± 7.5 |
56 ± 4.4 |
Lung metastases |
7 |
6.2 ± 2.1 |
50 ± 2.7 |
DCIS |
8 |
0.6 ± 0.02 |
47 ± 6.4 |
ME/CFS |
Table 1. NM23 Measurements in Breast Cancer Patient Serum
Data are mean ± SEM NM23 by ELISA. Diagnoses are based on the clinical history.
DCIS: Ductal Carcinoma in situ. ME/CFS: Myalgic Encephalomyelitis/Chronic Fatigue Syndrome
Effect of NM23 inhibition/P2Y1 receptor antagonism on tumor formation in SCID mice
Because extracellular NM23 can be expected to maintain levels of ATP/ADP in the bloodstream, we have proposed that extracellular Nm23 exerts its action through the activation of the endothelial purine nucleotide receptor P2Y1. This receptor displays an agonist rank order of ADP>ATP with no effect of adenosine. Ellagic acid (EA) is known to inhibit NM23activity [34] and has been shown to be anti-angiogenic [35,36]. MRS2179 is a potent endothelial P2Y1 receptor antagonist [37]. We applied MRS2179 (200 µM) and endostatin (200 mM) continually by mini-osmotic pumps (0.11 µl/hr) and EA in drinking water (120 µg/ml) to SCID mice carrying MDA-MB-231Luc+ tumors. Primary tumor growth was detected by measuring tumor volume and by non-invasive in vivo imaging (IVIS). The treatment showed a therapeutic effect in the suppression of tumor growth at week 16 as evidenced by the strong reduction of tumor volume in the treated groups (Figure 5; 2nd set-EA; 3rd set- MRS2179; 4th set-combination of MRS2179+EA),which were reduced by 60%, 65% and 68%, respectively (Figure 6). These treatments were comparable to the effect of endostatin (Figure 6) employed as a positive control. When these effects were examined longitudinally, treatments were seen to reduce primary tumor growth over time (Figure 6). Endostatin reduced primary tumor development at 11 wk by 73%, comparable to the effect of the combination of EA and MRS2179 treatment, which reduced primary tumor growth by 69% at the same time point (Figure 6 inset).
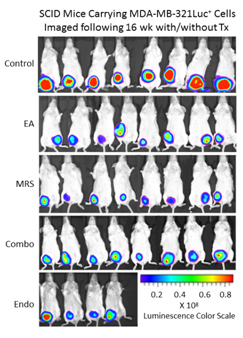
Figure 5. P2Y1 Receptor blockade or NM23 antagonist significantly suppresses tumor formation in vivo. Once MDA-MB-231Luc+ primary tumors in SCID mice attained a volume of 100-200 mm3, the osmotic pumps were implanted subcutaneously (back scruff) to deliver 200 mM MRS2179 or endostatin (pump internal volume, 100 μl) continuously delivered drugs at the rate of 0.11μl/hr for 30 days (26.4 pg/gram/day for both MRS 2179 and endostatin). EA was administrated in drinking water at 120 μg/ml (~34 mg/gram/day). Tumor volume and tumor bioluminescence were measured weekly to evaluate the effect of antitumor inhibitors (n=8 mice/group), and the images represent inhibition of primary tumor at 16 weeks compared to the control group (no treatment) at week 16 by bioluminescent activity.
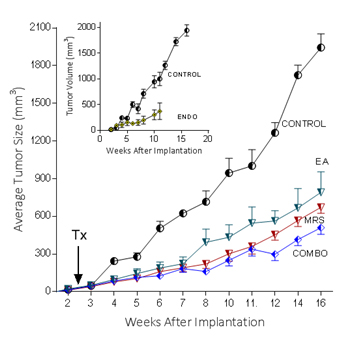
Figure 6. P2Y1 Receptor blockade or NM23 antagonist significantly suppresses tumor formation in vivo.
In separate experiments conducted as described for the images seen in figure 5, with 6 animals/group, the effect of endostatin, EA, MRS2179 or the combination on tumor volume over time was determined. Inset: comparison of control data versus endostatin treatment.
Effect of NM23 inhibition/P2Y1 receptor antagonism on tumor metastasis in SCID mice:
We detected metastases by imaging MDA-MB-231Luc+cell luciferase activity ex vivo in the lungs of mice carrying MDA-MB-231Luc+ tumors (Figure 6). This approach permits a quantitative assessment of lung metastases as bioluminescence after removal of the lungs imaged directly. As expected, significant lung tumor formation was apparent at week 16 shown as hundreds of millions of counts (Figure 7). Treatments significantly reduced the metastatic burden in the lungs as shown. MRS2179 and EA treatments both significantly lowered the metastatic burden in the lung (Figure 7) and EA was more efficacious that MRS2179. Further reduction was seen in the combination MRS2179/EA treatment, similar to that observed with endostatin treatment.
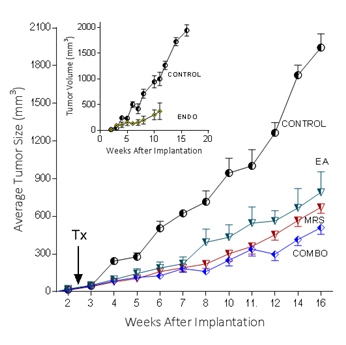
Figure 7. Suppression of MDA-MB-231 metastatic lesions by MRS2179 and EA. At 16 weeks, mice were sacrificed and lung, liver and pancreas tissues were removed and imaged for luciferase activity. Log2 bioluminescence was determined in lung tissues from 6 animals in each group. Metastatic tumor burden was reduced by MRS 2179 (MRS) or ellagic acid treatment (EA) and the combination treatment. Representative images of metastatic lesions seen in each treatment group are shown. Data are mean ± SEM, n=6-8; *p=<0.05, **p=<0.01.
Discussion
Here we examine the role of extracellular NM23 that appears to be very different from that of the intracellular expression of the kinase [25]. Evidence that NM23 appears in the conditioned media of breast cancer cells grown in culture is incontrovertible [38,39]. There are ten isoforms of this plurifunctional protein [40,41], only two of which (NDPK-A and -B) are known to appear extracellularly [21] as shown here in breast cancer patients. NM23 is distributed in cytosol, mitochondria, plasma membrane and the nucleus [42]. NM23 was originally described as non-metastatic 23 gene found in mouse melanoma cells as a homolog of the drosophila awd protein. Gene expression was thought to be inversely related to metastasis potential [14], although not exclusively [43-45]. Less attention however, was paid to NM23’s enzymatic function in maintaining ATP/ADP levels in the bloodstream. Nonetheless, the ability of circulating purine nucleotides to regulate blood flow locally [46,47] and modulate platelet aggregation [48] are known.
While we demonstrated the angiogenic activity of extracellular NM23nearly a decade ago [18] and more recently showed that NM23 activates the vascular endothelial growth factor receptor in support of angiogenesis [21], these actions of NM23 have recently been described in in vivo models of vasculogenesis by Weiland’s group [26], although these authors did not differentiate between an intracellular versus extracellular role of the kinase. Nevertheless, NM23 is now of significant interest in cancer biology; extracellular NDPK-A is a diagnostic biomarker for renal cell carcinoma [49], and increased expression is associated with pancreatic cancer [50]. These authors demonstrate that NM23 (NDPK-A) expression correlates with poor overall survival. It has also been demonstrated that intracellular NM23 (NDPK-B) is over expressed in hepatocellular carcinoma and NDPK-B shRNA inhibits tumor growth in xenotransplated tumors [51].
By focusing only on correlations between NM23 gene expression; cellular inactivation, or expression of a mutant enzyme intracellularly with cancer outcomes, we miss the additional role of extracellular NM23 in predicting the likelihood of progression to indolent disease in breast cancer. Our work shown here documenting extracellular secretion of NM23 by human breast cancer cells implanted in mice as well as our earlier work demonstrating an angiogenic effect of NM23 and the molecular basis of its trans-activation of VEGFR2, has set the stage for understanding the importance of NM23as an extracellular signal in tumorigenesis and may provide a survival factor in the metastatic niche. Our data do not necessarily refute the role of intracellular NM23 serving a metastasis suppressor, but rather extend a mechanistic understanding of the actions of extracellular NM23.
Preserving purine nucleotides in the blood stream is consistent with ATP/ADP levels activating endothelial purinergic receptors (P2Y1R). P2YR are recognized [52] as regulators of carcinogenesis and endothelial cell functions and are modulators of platelet aggregation and blood flow regulation [10,53]. Extracellular ATP and ADP activate endothelial cell P2Y1 receptors to release vasoactive mediators such as nitric oxide, prostacyclin, and additional ATP [10,47] to elicit vasorelaxant, proliferative and angiogenic effects [54]. Human endothelial P2Y1 receptors trans-activate endothelial VEGFR-2, suggesting a direct link between extracellular nucleotide generation and growth factor signaling [21,26,55] that fits with our hypothesis since in the absence of VEGF, inhibition of NM23by ellagic acid will prevent VEGFR-2 activation as we [18,21,34] and others [56] have shown.
A role for extracellular NM23 in the process of metastasis is supported by disruption of CD39 (ecto-apyrase; EC3.6.1.5), the dominant vascular ecto-nucleotidase which has been observed to inhibit tumor angiogenesis and metastasis [57,58]. This would lead to decreased ADP levels in capillary vessels and decreased P2Y1R activation. As we document here, human breast carcinoma cells implanted in the mammary fat of immunocompromised mice secrete NM23 measurable in the serum. We propose that NM23 makes the metastatic niche receptive by endothelial activation and predicts the likelihood of progression to indolent disease by supporting dissemination and angiogenesis [21]. Our hypothesis is not informed by women taking clopidogrel, a platelet P2Y12 antagonist [59] that does not block endothelial P2Y1 receptors.
By collecting the blood at different stages of primary tumor growth we determined that NM23 levels were high from the first time point of tumor formation and trended toward dramatic accumulation by week 16 (Figure 1). There is a positive correlation (r2=0.85) between secreted NM23 and tumor size consistent with its role in metastatic progression (Figure 1C). NM23was found in the serum of patients with breast cancer (Figure 2) and NM23 levels were elevated in women with metastatic breast cancer, lower in patients with DCIS, and all but absent in women without cancer. We have suggested that elaboration of NM23 by breast tumor cells may be a general phenomenon. We show that, in the nu/nu immunocompromised mouse, NM23 is found in the serum of mice carrying the inflammatory breast cancer cell type Mary-X [60] and the bronchioloalveolar cell carcinoma(BAC) (Figure 3b). We did not observe a clear correlation across the comparison of NM23 protein in the serum and mRNA expression in the tumors (Figure 3), although the highest message expression in the Mary-X tumors fits with the highest levels found in serum. The relevance of finding NM23 levels in the serum of animals carrying the Mary-X or BAC tumors together with the finding of NM23 in bronchial lavage fluid in patients with bronchial system tumors [61] is that extracellular NM23 may play a role in tumorigenesis in other cancers.
Our data are supported by the Oncomine database, demonstrating that upregulated NM23 mRNA expression positively correlated with poor prognosis in breast cancer patients (Figure 4a,4b). NM23 mRNA expression is also strongly correlated with poor overall survival and relapse-free survival in breast cancer patients (Figure 4c,4d). Given the impact of NM23 on extracellular transactivation of VEGFR, which is fundamental to breast cancer angiogenesis and metastasis, our data suggest NM23 as a possible therapeutic target in breast cancer management. If increased gene expression in breast cancer patient’s means increased extracellular elaboration of NM23, then the public database supports our hypothesis by providing an unbiased positive correlation of mRNA expression and breast cancer that fits with our notion of NM23 secretion in experimental breast cancer and that detected in human breast cancer patients (Figure 1-3).
The ability to image MDA-MB-231Luc+ tumors in SCID mice clearly shows the effect of blocking the NM23:P2Y1R pathway at 16 weeks of tumor growth (Figure 5). When followed over time, the development of the primary MDA-MB-231Luc+ tumors is suppressed by the treatment of mice with the NM23 inhibitor ellagic acid [62], or the P2Y1 antagonist MRS2179 [18]. Primary tumor suppression is not significantly improved with the combination of NM23 and MRS2179 (Figure 6) perhaps because either P2Y1 or NM23 blockade would accomplish the same result. When these treatments are compared at the time of sacrifice at the end of the study, there is a clear reduction on the presence of lung metastases (Figure 6). Comparisons to endostatin, known to prevent metastases in the mouse [63,64] and now known to possess ATPase activity [65] consistent with an action in the NM23:P2Y1R pathway, are encouraging offering both a comparative benefit as well as suggesting a mechanistic role for endostatin consistent with our hypothesis.
Acknowlegements
Our research was supported by National Institutes of Health grants HD 053028 and GM 104944, and a grant from the Bill and Melinda Gates Foundation to ILOB. Institutional support was provided by INBRE grant P20 RR-016464 and NIH 8 P20 GM103440. SN was supported by a Fellowship from the Mick Hitchcock, PhD Research Fund.
References
- Schmidt-Kittler O, Ragg T, Daskalakis A, Granzow M, Ahr A, et al. (2003) From latent disseminated cells to overt metastasis: genetic analysis of systemic breast cancer progression. Proc Natl Acad Sci U S A 100: 7737-7742. [Crossref]
- Demicheli R, Retsky MW, Hrushesky WJ, Baum M, Gukas ID (2008) The effects of surgery on tumor growth: a century of investigations. Ann Oncol 19: 1821-1828. [Crossref]
- Retsky M, Demicheli R, Hrushesky W, Baum M, Gukas I (2010) Surgery triggers outgrowth of latent distant disease in breast cancer: an inconvenient truth? Cancers (Basel) 2: 305-337. [Crossref]
- Hagemeister FB Jr, Buzdar AU, Luna MA, Blumenschein GR (1980) Causes of death in breast cancer: a clinicopathologic study. Cancer 46: 162-167. [Crossref]
- van Zijl F, Krupitza G, Mikulits W (2011) Initial steps of metastasis: cell invasion and endothelial transmigration. Mutat Res 728: 23-34. [Crossref]
- Mack GS, Marshall A (2010) Lost in migration. Nat Biotechnol 28: 214-229. [Crossref]
- Thiery JP, Acloque H, Huang RY, Nieto MA (2009) Epithelial-mesenchymal transitions in development and disease. Cell 139: 871-890. [Crossref]
- Tomaskovic-Crook E, Thompson EW, Thiery JP (2009) Epithelial to mesenchymal transition and breast cancer. Breast Cancer Res 11: 213. [Crossref]
- Moustakas A, Heldin CH (2012) Induction of epithelial-mesenchymal transition by transforming growth factor β. Semin Cancer Biol 22: 446-454. [Crossref]
- Buxton IL, Kaiser RA, Oxhorn BC, Cheek DJ (2001) Evidence supporting the Nucleotide Axis Hypothesis: ATP release and metabolism by coronary endothelium. Am J Physiol Heart Circ Physiol 281: H1657-1666. [Crossref]
- Attwood PV, Wieland T (2015) Nucleoside diphosphate kinase as protein histidine kinase. Naunyn Schmiedebergs Arch Pharmacol 388: 153-160. [Crossref]
- Postel EH (2003) Multiple biochemical activities of NM23/NDP kinase in gene regulation. J Bioenerg Biomembr 35: 31-40. [Crossref]
- Dammai V, Adryan B, Lavenburg KR, Hsu T (2003) Drosophila awd, the homolog of human nm23, regulates FGF receptor levels and functions synergistically with shi/dynamin during tracheal development. Genes Dev 17: 2812-2824. [Crossref]
- Freije JM, MacDonald NJ, Steeg PS (1998) Nm23 and tumour metastasis: basic and translational advances. Biochem Soc Symp 63: 261-271. [Crossref]
- de la Rosa A, Mikhak B, Steeg PS (1996) Identification and characterization of the promoter for the human metastasis suppressor gene nm23-H1. Arch Med Res 27: 395-401. [Crossref]
- Okabe-Kado J, Kasukabe T (2003) Physiological and pathological relevance of extracellular NM23/NDP kinases. J Bioenerg Biomembr 35: 89-93. [Crossref]
- Okabe-Kado J, Kasukabe T, Honma Y, Hanada R, Nakagawara A, et al. (2005) Clinical significance of serum NM23-H1 protein in neuroblastoma. Cancer Sci 96: 653-660. [Crossref]
- Rumjahn SM, Javed MA, Wong N, Law WE, Buxton IL (2007) Purinergic regulation of angiogenesis by human breast carcinoma-secreted nucleoside diphosphate kinase. Br J Cancer 97: 1372-1380. [Crossref]
- Rumjahn SM, Yokdang N, Baldwin KA, Thai J, Buxton IL (2009) Purinergic regulation of vascular endothelial growth factor signaling in angiogenesis. Br J Cancer 100: 1465-1470. [Crossref]
- Yokdang N, Buxton ND, Buxton IL (2009) Measurement of human breast tumor cell-secreted shNDPK-B in a murine breast cancer model suggests its role in metastatic progression. Proc West Pharmacol Soc 52: 88-91. [Crossref]
- Yokdang N, Tellez JD, Tian H, Norvell J, Barsky SH, et al. (2011) A role for nucleotides in support of breast cancer angiogenesis: heterologous receptor signalling. Br J Cancer 104: 1628-1640. [Crossref]
- Iiizumi M, Bandyopadhyay S, Pai SK, Watabe M, Hirota S, et al. (2008) RhoC promotes metastasis via activation of the Pyk2 pathway in prostate cancer. Cancer Res 68: 7613-7620. [Crossref]
- Smagghe BJ, Stewart AK, Carter MG, Shelton LM, Bernier KJ, et al. (2013) MUC1* ligand, NM23-H1, is a novel growth factor that maintains human stem cells in a more naïve state. PLoS One 8: e58601. [Crossref]
- Thirkill TL, Cao T, Stout M, Blankenship TN, Barakat A, et al. (2007) MUC1 is involved in trophoblast transendothelial migration. Biochim Biophys Acta 1773: 1007-1014. [Crossref]
- Marino N, Nakayama J, Collins JW, Steeg PS (2012) Insights into the biology and prevention of tumor metastasis provided by the Nm23 metastasis suppressor gene. Cancer Metastasis Rev 31: 593-603. [Crossref]
- Feng Y, Gross S, Wolf NM, Butenschon VM, Qiu Y, et al. (2014) Nucleoside diphosphate kinase B regulates angiogenesis through modulation of vascular endothelial growth factor receptor type 2 and endothelial adherens junction proteins. Arterioscler Thromb Vasc Biol 34: 2292-2300. [Crossref]
- Xiao Y, Ye Y, Yearsley K, Jones S, Barsky SH (2008) The lymphovascular embolus of inflammatory breast cancer expresses a stem cell-like phenotype. Am J Pathol 173: 561-574. [Crossref]
- Liu B, Feng Y, Zhang JY, Li HM, Li XD, et al. (2013) Imaging of bronchioloalveolar carcinoma in the mice with the αⅤβ3 integrin-targeted tracer (99m)Tc-RGD-4CK. Transl Res 162: 174-180. [Crossref]
- Curtis C, Shah SP, Chin S-F, Turashvili G, Rueda OM, et al. (2012) The genomic and transcriptomic architecture of 2,000 breast tumours reveals novel subgroups. Nature 486: 346-352. [Crossref]
- Györffy B, Lanczky A, Eklund AC, Denkert C, Budczies J, et al. (2010) An online survival analysis tool to rapidly assess the effect of 22,277 genes on breast cancer prognosis using microarray data of 1,809 patients. Breast Cancer Res Treat 123: 725-731. [Crossref]
- Hamby CV, Abbi R, Prasad N, Stauffer C, Thomson J, et al. (2000) Expression of a catalytically inactive H118Y mutant of nm23-H2 suppresses the metastatic potential of line IV Cl 1 human melanoma cells. Int J Cancer 88: 547-553. [Crossref]
- Hippe HJ, Lutz S, Cuello F, Knorr K, Vogt A, et al. (2003) Activation of heterotrimeric G proteins by a high energy phosphate transfer via nucleoside diphosphate kinase (NDPK) B and Gbeta subunits. Specific activation of Gsalpha by an NDPK B.Gbetagamma complex in H10 cells. J Biol Chem 278: 7227-7233. [Crossref]
- Eltzschig HK, Weissmüller T, Mager A, Eckle T (2006) Nucleotide metabolism and cell-cell interactions. Methods Mol Biol 341: 73-87. [Crossref]
- Buxton IL (2008) Inhibition of Nm23H2 gene product (NDPK-B) by angiostatin, polyphenols and nucleoside analogs. Proc West Pharmacol Soc 51: 30-34. [Crossref]
- Cao Y, Cao R (1999) Angiogenesis inhibited by drinking tea. Nature 398: 381. [Crossref]
- Singh AK, Seth P, Anthony P, Husain MM, Madhavan S, et al. (2002) Green tea constituent epigallocatechin-3-gallate inhibits angiogenic differentiation of human endothelial cells. Arch Biochem Biophys 401: 29-37. [Crossref]
- Shen J, DiCorleto PE (2008) ADP stimulates human endothelial cell migration via P2Y1 nucleotide receptor-mediated mitogen-activated protein kinase pathways. Circ Res 102: 448-456. [Crossref]
- Buxton IL, Yokdang N (2011) Extracellular NM23 Signaling in Breast Cancer: Incommodus Verum. Cancers (Basel) 3: 2844-2857. [Crossref]
- Yegutkin GG (2014) Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol 49: 473-497. [Crossref]
- Lacombe ML, Milon L, Munier A, Mehus JG, Lambeth DO (2000) The human Nm23/nucleoside diphosphate kinases. J Bioenerg Biomembr 32: 247-258. [Crossref]
- McDermott WG, Boissan M, Lacombe ML, Steeg PS, Horak CE (2008) Nm23-H1 homologs suppress tumor cell motility and anchorage independent growth. Clin Exp Metastasis 25: 131-138. [Crossref]
- Bertheau P, De La Rosa A, Steeg PS, Merino MJ (1994) NM23 protein in neoplastic and nonneoplastic thyroid tissues. Am J Pathol 145: 26-32. [Crossref]
- Hailat N, Keim DR, Melhem RF, Zhu XX, Eckerskorn C, et al. (1991) High levels of p19/nm23 protein in neuroblastoma are associated with advanced stage disease and with N-myc gene amplification. J Clin Invest 88: 341-345. [Crossref]
- Keim D, Hailat N, Melhem R, Zhu XX, Lascu I, et al. (1992) Proliferation-related expression of p19/nm23 nucleoside diphosphate kinase. J Clin Invest 89: 919-924. [Crossref]
- Melhem RF, Zhu XX, Hailat N, Strahler JR, Hanash SM (1991) Characterization of the gene for a proliferation-related phosphoprotein (oncoprotein 18) expressed in high amounts in acute leukemia. J Biol Chem 266: 17747-17753. [Crossref]
- Buxton ILO, Cheek DJ (1995) On the origin of extracellular ATP in cardiac blood vessels: A dual role for endothelium. In: Belardinelli L, Pelleg A. Adenosine Adenine Nucleotides from Mol Biol to Integr Physiol. New York: Kluwer: 193-197.
- Yang S, Cheek DJ, Westfall DP, Buxton IL (1994) Purinergic axis in cardiac blood vessels. Agonist-mediated release of ATP from cardiac endothelial cells. Circ Res 74: 401-407. [Crossref]
- Gachet C (2006) Regulation of platelet functions by P2 receptors. Annu Rev Pharmacol Toxicol 46: 277-300. [Crossref]
- Su Kim D, Choi YD, Moon M, Kang S, Lim JB, et al. (2013) Composite three-marker assay for early detection of kidney cancer. Cancer Epidemiol Biomarkers Prev 22: 390-398. [Crossref]
- Takadate T, Onogawa T, Fujii K, Motoi F, Mikami S, et al. (2012) Nm23/nucleoside diphosphate kinase-A as a potent prognostic marker in invasive pancreatic ductal carcinoma identified by proteomic analysis of laser micro-dissected formalin-fixed paraffin-embedded tissue. Clin Proteomics 9: 8. [Crossref]
- Lee MJ, Xu DY, Li H, Yu GR, Leem SH, et al. (2012) Pro-oncogenic potential of NM23-H2 in hepatocellular carcinoma. Exp Mol Med 44: 214-224. [Crossref]
- White N, Burnstock G (2006) P2 receptors and cancer. Trends Pharmacol Sci 27: 211-217. [Crossref]
- Burnstock G (2006) Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev 58: 58-86. [Crossref]
- Kashiwagi S, Izumi Y, Gohongi T, Demou ZN, Xu L, et al. (2005) NO mediates mural cell recruitment and vessel morphogenesis in murine melanomas and tissue-engineered blood vessels. J Clin Invest 115: 1816-1827. [Crossref]
- Seye CI, Yu N, González FA, Erb L, Weisman GA (2004) The P2Y2 nucleotide receptor mediates vascular cell adhesion molecule-1 expression through interaction with VEGF receptor-2 (KDR/Flk-1). J Biol Chem 279: 35679-35686. [Crossref]
- Wang N, Wang ZY, Mo SL, Loo TY, Wang DM, et al. (2012) Ellagic acid, a phenolic compound, exerts anti-angiogenesis effects via VEGFR-2 signaling pathway in breast cancer. Breast Cancer Res Treat 134: 943-955. [Crossref]
- Goepfert C, Sundberg C, Sévigny J, Enjyoji K, Hoshi T, et al. (2001) Disordered cellular migration and angiogenesis in cd39-null mice. Circulation 104: 3109-3115. [Crossref]
- Jackson SW, Hoshi T, Wu Y, Sun X, Enjyoji K, et al. (2007) Disordered purinergic signaling inhibits pathological angiogenesis in cd39/Entpd1-null mice. Am J Pathol 171: 1395-1404. [Crossref]
- Dol-Gleizes F, Marés AM, Savi P, Herbert JM (1999) Relaxant effect of 2-methyl-thio-adenosine diphosphate on rat thoracic aorta: effect of clopidogrel. Eur J Pharmacol 367: 247-253. [Crossref]
- Alpaugh ML, Tomlinson JS, Shao ZM, Barsky SH (1999) A novel human xenograft model of inflammatory breast cancer. Cancer Res 59: 5079-5084. [Crossref]
- Huwer H, Kalweit G, Engel M, Welter C, Dooley S, et al. (1997) Expression of the candidate tumor suppressor gene nm23 in the bronchial system of patients with squamous cell lung cancer. Eur J Cardiothorac Surg 11: 206-209. [Crossref]
- Malmquist NA, Anzinger JJ, Hirzel D, Buxton IL (2001) Ellagic acid inhibits nucleoside diphosphate kinase-B activity. Proc West Pharmacol Soc 44: 57-59. [Crossref]
- Liby K, Neltner B, Mohamet L, Burd C, Ben-Jonathan N (2003) Endostatin expression by MDA-MB-435 breast cancer cells effectively inhibits tumor growth. Cancer Biol Ther 2: 48-52. [Crossref]
- Indraccolo S, Gola E, Rosato A, Minuzzo S, Habeler W, et al. (2002) Differential effects of angiostatin, endostatin and interferon-alpha(1) gene transfer on in vivo growth of human breast cancer cells. Gene Ther 9: 867-878. [Crossref]
- Wang S, Lu XA, Liu P, Fu Y, Jia L, et al. (2015) Endostatin has ATPase activity, which mediates its antiangiogenic and antitumor activities. Mol Cancer Ther 14: 1192-1201. [Crossref]







