Purpose: Aerobic training and beta blockers may manipulate adrenoceptors number. Therefore, the present study examined the effect of aerobic exercise training on the adrenoceptors number in coronary artery disease patients (CAD) treated with atenolol b-blocker.
Methods: Sixty patients: 30 males and 30 women with diagnosed CAD were treated for twelve weeks with atenolol b-blocker. Following the 12 weeks atenolol treatment, patients were divided randomly into four groups: 15 males aged (years) 46.6±2.0 (MTR) and 15 trained females 47.3±2.0 (FTR) were assigned for 12 weeks aerobically in addition to the atenolol while, 15 males 46.8±2.0 (MC) and 15 women 46.9±2.0 (FC) treated only with atenolol served as controls. Adrenoceptors were measured by the specific binding 3H-CGP 12177.
Results: Following atenolol treatment, all groups significantly (p<0.05) reduced adrenoceptor levels (MTR 3.50±0.17 to 3.07±0.19, FTR 3.42±0.16 to 3.09±0.15, MC 3.49±0.14 to 3.07±0.19 and FC 3.44±0.15 to 3.05±0.14 fmol/106). Following aerobic training, levels of adrenoceptors rose significantly (p<0.05) in the MTR from 3.07±0.19 to 3.74 ±0.14, and FTR from 3.09±0.15 to 3.69±0.16 whereas in the MC and FC groups it remained unchanged (3.07±0.19 and 3.05 fmol/106 respectively).
Conclusions: Data suggest that in males and females regular and habitual moderate intensity aerobic exercise combined with atenolol b-blocker treatment may help to counter the b-blocker related decline in the b-adrenergic receptors number.
Beta adrenergic receptors, aerobic exercise, atenolol, maximal oxygen uptake, catecholamine.
Coronary artery disease (CAD) patients have a reduced exercise tolerance and a decreased left ventricle inotropic reserve related to increased vascular afterload, arterial-ventricular load mismatching, physical deconditioning and impaired autonomic regulation. The outcomes are abnormalities that result in neuro-hormonal activation and autonomic imbalance with increase in sympathetic activity and withdrawal of vagal activity [1]. This triggers responses that are compensatory at first, but eventually become part of the disease process itself, leading to further worsening cardiac function [2]. Among these responses is the activation of the sympathetic nervous system that provides inotropic support to increase left ventricular contractility [3].
CAD is associated with changes in the sympathetic nervous system. Indeed, mounting evidence indicates that adrenergic receptors are functionally involved in numerous processes underlying cardiovascular disorders [4].
Drugs that selectively block adrenergic activity regulate heart rate, myocardial contraction force and vascular resistance [5,6]. However, some beta-blockers possess attenuation of human lymphocytic adrenoceptors [7] and hence, offset the β-adrenergic cascade [8]. This may alter catecholamine regulation on sensitive tissue and post-receptor mechanisms, [9] since the interface between the sympathetic fibers and the cardiovascular system is formed by the adrenoceptors [2].
Aerobic training has been shown to up-regulate the β-adrenergic-receptor number on mononuclear lymphocytes [9]. Thus, the administration of atenolol b-blocker may attenuate or blunt the decrease of adrenoceptors while increasing left ventricular contractility compared to other beta blockers agents [10]. Therefore, the purpose of the present study was twofold: 1) to examine the effect of an aerobic exercise training program on the number of adrenoceptors in coronary artery disease patients treated with atenolol b-blocker, and 2) to assess whether continuation of medication intervention without exercise supplementation will further adversely affect the function of the sympathoadrenergic system.
Subjects: Sixty patients (ntotal=60), 30 males and 30 women, with diagnosed CAD were treated for 12 weeks with atenolol b-blocker. Following 12 weeks of atenolol b-blocker treatment, patients were divided randomly into four groups. Group 1 included 15 trained males (MTR) aged 46.6±2.0 years and group 2 included 15 trained females (FTR) 47.3±2.0 years. Groups 1 and 2 completed 12 weeks of aerobic exercise in addition to atenolol treatment. Group 3 included 15 males (MC) aged 46.8±2.0 years and group 4 included 15 women (FC) 46.9±2.0 years, treated only with atenolol and served as controls. A written consent form was obtained from each subject, approved by the Clinical Science Center Committee on Human Subjects. Patients had prior myocardial infarction or CAD documented within one year of the study, by clinical and electrocardiographic criteria and cardiac catheterization revealing in the control group: 14 patients with single-vessel disease, double-vessel disease in 10, three-vessel disease in 2, inferior wall motion abnormalities noted in 2, and posterior wall motion abnormalities in 2. In the exercising group: 8 patients with single-vessel disease, double-vessel disease in 12, three-vessel disease in 4, inferior wall motion abnormalities noted in 2, posterior in 2 and anterior in 2. All patients took 25 mg•day-1 of atenolol b-adrenergic blocking agent. Six patients (3 males and 1 female from the experimental groups) took ACE-inhibitors and 8 took diuretics (2 males and 2 females from the experimental groups). They were asked to withdraw from these medications during the week prior to blood sampling, in order to enable sufficient washout period for these drugs. Patients in the present study were not included if they had mitral regurgitation, rhythm abnormalities, systemic hypertension or left ventricular hypertrophy, history of auto-immunity, cancer, diabetes and other endocrinopathy. Patients were not active participants in supervised aerobic programs.
Experimental design: Following baseline measurements of blood samples, in the first stage of the study (onset of medication) all patients participating in the study were treated with atenolol b-blocker, and assessed for the effect of the drug on the adrenoceptors number. The following stage included 12 weeks of aerobic training for the experimental group and no training for the controls. Accordingly, four groups were established randomly: two exercising groups of males and females, and two control groups of males and females.
There were 3 testing sessions for all subjects. The first session was devoted to familiarizing the subjects to the treadmill and explaining the nature of the study. Adipose fat assessment included measurement of total body weight [±0.05 kg], skin fold thicknesses at 7 sites (±1 mm) using the Lange Caliper (chest, axilla, triceps, sub-scapula, abdomen, suprailium, front thigh and circumferences at the shoulder). Anthropometric procedures followed the recommendations of Behnke and Wilmore.11
After 12 weeks of taking atenolol b-blocker (session two), following warm-up, patients underwent a Balke treadmill stress test, according to the guidelines of the American College of Sports Medicine.12 Training workload (training heart rate) was calculated for each patient according to the Karvonen equation.13 At the third session following twelve weeks of training, a peripheral venous blood sample was taken for analysis, and a stress test was given to all participants in the study.
Blood sampling: Peripheral venous blood samples (2.5 mL) were collected by sterile antecubital venipuncture techniques with the samples analyzed within 6 hours. Time of day for blood sampling was kept consistent to control for problems associated with diurnal variation. Blood collection was obtained, 1) before onset of drug intervention, 2) after 12 weeks of drug intervention, and 3) following 12 weeks of exercise training during drug intervention (treatment group) or 12 weeks of no exercise training during continued drug intervention (control group).
Receptor assay. 3H-CGP 12177 was used to determine the density of adrenergic receptors. A more detailed description of the synthesis can be found elsewhere [14-16]. 3H-CGP 12177, had a specific activity of 54 Ci/mmol. The assay buffer was the same as the Tris-MgCl2 buffer used for membrane preparation. In four to eight tubes (depending on the amount of protein present in each suspension), 300 µL of membrane homogenates were prepared to a final volume of 2 mL. Increasing concentrations of 3H-CGP 12177 (from 0.2 to 3 nM) were added. The incubation conditions (370C for 60 minutes) ensured that equilibrium had been reached between the receptors and the radioligand. The reaction was terminated by rapid vacuum filtration through Whatman GF/C filters; filters were washed with a 15-mL excess of ice-cold buffer. These filters were then dried and placed into 5 mL scintillation fluid (Insta-Gel, Packard). A liquid scintillation counter was used to determine the sample radioactivity (Packard SL 2000, 80% efficiency). Nonspecific binding was detected in the presence of 3 1£M of propanolol and averaged 10-15% of the total. Maximum density (Bmax) and apparent affinity (Kd) of binding sites were assessed in each individual experiment using a nonlinear least squares regression program.
Training program: After medication intervention, the exercising groups were closely supervised during training. Sessions were held 3-4 times per week for 12 weeks. Subjects ran or walked for 45 minutes at a mean heart rate of 124±10 beat • min-1 which corresponded to 65% of the maximal work capacity (HRR) of each subject. Training loads were adjusted as work capacity at the prescribed heart rate increased as a result of training.
Statistical methods: Each variable was analyzed by MANOVA with repeated measures on the stage factor. The criterion for rejection of the null hypothesis was set at alpha = 0.05. Tukey’s HSD procedure was utilized for specific post-hoc comparisons.
Mean descriptive and physiological variables data for the experimental and control groups are presented in Table 1. It reveals that at base MTR and MC compared to the FTR and FC groups, demonstrated significantly (p< 0.05) higher values for weight, height, heart rate, systolic, diastolic and mean arterial blood pressures. However, no significant differences were noted between the MTR group and MC group as well between the FTR and FC patients. Table 2 reports on the physiological changes following 12 weeks of atenolol administration and aerobic training. It shows that following aerobic training, patients in groups MTR and FTR compared to the MC and FC groups reduced significantly (p< 0.05), body fat percentage, heart rate, systolic, diastolic and mean blood pressures. Maximal oxygen uptake rose in the MTR and FTR exercising groups compared to the untrained patients (from 39.1±2.3 to 45.6± 2.3 and 35.6±2.7 to 41.0±2.9 mL•kg-1•min-1 respectively) while in the MC and FC it remained unchanged. Following atenolol treatment, MC and FC, reduced significantly (p<0.05) adrenoceptor levels (3.49±0.14 to 3.07±0.19 and 3.44±0.15 to 3.05±0.14 fmol/106 respectively). Following aerobic training period, levels of adrenoceptors in the MC and FC groups remained unchanged (3.07±0.19 and 3.05 fmol/106 respectively). Figure 1, reveals that in the MTR and FTR groups after 12 weeks of drug intervention, levels of adrenoceptors number were significantly decreased (p< 0.001), compared to the values measured before the onset of drug intervention. Following 12 weeks of exercise training in conjunction with drug intervention, both groups increased significantly (p<0.05) adrenoceptors values above the base, before exercise intervention.
Table 1. Physiological variables following 12 weeks of atenolol administration in male and female CAD patients (MC and FC; controls) and 12 weeks of aerobic training and atenolol administration in male and female CAD patients (MTR and FTR) (mean ± S.D).
Variable |
MTR |
FTR |
MC |
FC |
N of Subjects |
15 |
15 |
15 |
15 |
Age (years) |
46.6 ± 2.0 |
47.3 ± 2.0 |
46.8 ± 2.0 |
46.9 ± 2.0 |
Weight (kg) |
74.2 ± 2.1 |
57.4 ± 2.3a |
73.4 ± 2.5 |
56.8 ± 2.2a |
Height (cm) |
178.3 ± 2.7 |
167.5 ± 2.4a |
178.9 ± 2.2 |
167.7 ± 2.0a |
Fat (%) |
19.6 ± 3.2 |
20.9 ± 3.8 |
20.1 ± 3.5 |
22.1 ± 4.0 |
Heart rate (beats•min-1) |
76.2 ± 4.9 |
79.5 ± 3.9 |
77.2 ± 4.9 |
78.7 ± 3.9 |
Systolic blood p (mmHg) |
119.8 ± 8.5 |
110.7 ± 7.1a |
121.1 ± 7.8 |
112.1 ± 6.9 a |
DBP (mmHg) |
80.2 ± 4.9 |
74.3 ± 3.2 a |
82.1 ± 5.1 |
74.7 ± 3.6 a |
MABP (mmHg) |
93.4 ± 3.0 |
86.4 ± 2.9 a |
94.4 ± 3.1 |
87.2 ± 3.0 a |
VO2 max (mL•kg-1•min-1) |
38.1 ± 2.3 |
36.0 ± 2.7 |
38.4 ± 2.4 |
36.7 ± 2.2 |
Table 2. Physiological variables following 12 weeks of atenolol administration in male and female CAD patients (MC and FC; controls) and 12 weeks of aerobic training and atenolol administration in male and female CAD patients (MTR and FTR) (mean ± S.D).
Variable |
MTR |
FTR |
MC |
FC |
N of subjects |
15 |
15 |
15 |
15 |
Weight (kg) |
70.6 ± 2.7b |
53.5 ± 2.3ab |
73.5 ± 2.5 |
56.6 ± 2.4a |
Body Fat (%) |
16.1 ± 2.6b |
16.9 ± 2.5b |
20.3 ± 3.6 |
21.8 ± 3.7 |
HR (beats•min-1) |
72.2 ± 4.9 |
74.3 ± 3.9 |
78.2 ± 4.6 |
80.3 ± 4.1 |
SBP (mmHg) |
117.8 ± 6.4 |
110.1 ± 5.0a |
120.9 ± 6.1 |
112.4 ± 5.9a |
DBP (mmHg) |
79.1 ± 3.2 |
72.9 ± 2.2a |
82.2 ± 3.8 |
73.9 ± 3.1a |
MABP (mmHg) |
92.0 ± 3.1 |
85.3 ± 3.1a |
95.1 ± 3.3 |
86.7 ± 3.0a |
VO2 max (mL•kg-1•min-1) |
45.6 ± 2.3 |
41.0 ± 2.9ab |
38.3 ± 2.5 |
36.5 ± 2.6 |
- Significant difference between males and females.
- Significant difference following aerobic training.
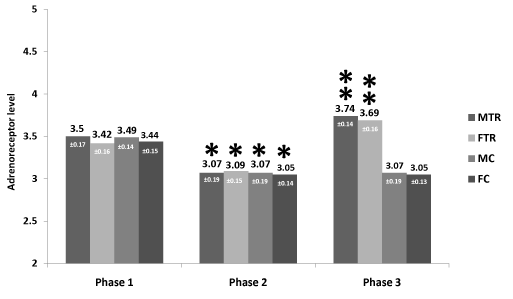
Figure 1. Effects of exercise training and atenolol on adrenoceptors (mean ± SD) in exercising males (MTR) and females (FTR). Before onset of drug intervention (phase 1), b. after 12 weeks of drug intervention (phase 2), and c. after 12 weeks of exercise training during drug intervention (phase 3). The asterisk signifies an interaction effect (p<0.05).
* = significant differences between phase 1 and phase 2
** = significant differences between phase 2 and phase 3
In the present study patients with CAD were treated with atenolol which is a cardioselective beta-blocker with potent activity against beta-1 adrenergic receptors found in cardiac muscle, but has little or no activity against beta-2 adrenergic receptors. It is widely used in hypertension, angina and for management of acute myocardial infarction. It can also help prevent myocardial infarction or myocardial damage after a myocardial infarction. Atenolol blocks the action of the sympathetic nervous system of the heart, thus reducing stress on the heart. However, the interplay between oxygen delivery and extraction is kept, suggesting a link between cardiac hemodynamic responses and skeletal muscle metabolic adaptations [17].
In the present study, 12 weeks of atenolol b-blocker administration resulted in significant reduction of adrenoceptors in all groups. Following aerobic training, the increase in adrenoceptors in the exercising groups, clearly illustrate benefits of an aerobic exercise program for CAD patients. Second, continuation of medication intervention in the untrained group did not further reduce the number of the b-adrenergic receptors.
Administration of b-blocker drugs is associated with a subnormal exercise-induced up-regulation and decreased functioning of the lymphocytic beta-adrenoceptors, while exercise-provoked up-regulation and improved functioning of beta-adrenoceptors which is blunted in cardiac patients receiving beta-adrenergic blockers [18]. Thus, any decrease in the number of b-adrenergic receptors will automatically lead to a reduction in functional capacity of the sympathetic nervous system and the responsiveness of b-adrenergic receptors [19,20].
These results are in agreement with previous work [21] which demonstrated that high aerobic capacity achieved following aerobic exercise training is associated with an increased density and ability of the b-adrenoceptors, in response to the significant increase in catecholamine levels during exercise. Catecholamine plays an essential role in the activation of the cardiovascular system and in the regulation of energy metabolism in a variety of physiological conditions. Many of these effects are mediated through b-adrenoceptors located on cell membranes [18]. Both short- and long-term aerobic exercise training induces a rapid up-regulation and more effective functioning and responsiveness in human b-adrenoceptors.
Maximal oxygen uptake rose in the exercising groups compared to the untrained groups. These results following 12 weeks of exercise training at 65% HRR were similar to those seen previously in young adults and elderly [22].
The decrease in adrenoceptor number following 12 weeks of medication treatment, indicates the duration of medication and the type of medication used (atenolol) are factors affecting the adrenoceptor number. The mechanism, in which atenolol administration may affect adrenoceptor number, may be by reducing IL-2 production that changes the intrinsic properties of lymphocyte cells [23]. Since b-blockers induce a marked and lasting decrease in the adrenoceptor number,5 they can potentially attenuate ability of the sympathetic system [24,25].
Data suggest that males and females participating in twelve weeks of regular habitual vigorous (65% HRR) aerobic exercise in conjunction with drug intervention may counter the atenolol b-blocker related decline in the b-adrenergic receptors number, thus, possess statistically significant higher adrenoceptor number than their untrained counterparts. The increased level of adrenoceptors following aerobic training program, may be due to the acute increases in concentrations of catecholamine in plasma during exercise which in turn, may increase b-adrenoceptors density. In addition, the current design does not enable us to comment on how long the effects of exercise last in patients taking atenolol b-blocker. However, we recommend patients keep exercising as long as they are treated with atenolol b-blocker, since the benefits of exercise are clear.
2021 Copyright OAT. All rights reserv
- Florea VG, Cohn JN (2014) The autonomic nervous system and heart failure. Circ Res 114: 1815-1826. [Crossref]
- Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, et al. (2009) The sympathetic nervous system in heart failure physiology, pathophysiology, and clinical implications. J Am Coll Cardiol 54: 1747-1762. [Crossref]
- Adams KF (2004) Pathophysiologic role of the renin-angiotensin-aldosterone and sympathetic nervous systems in heart failure. Am J Health Syst Pharm 61 Suppl 2: S4-13.
- Ferrara N, Komici K, Corbi G, Pagano G, Furgi G, et al. (2014) ß-adrenergic receptor responsiveness in aging heart and clinical implications. Front Physiol 4: 396.
- Leosco D, Rengo G, Iaccarino G, Filippelli A, Lymperopoulos A, et al. (2007) Exercise training and beta-blocker treatment ameliorate age-dependent impairment of beta-adrenergic receptor signaling and enhance cardiac responsiveness to adrenergic stimulation. Am J Physiol Heart Circ Physiol 293: H1596-603.
- Leosco D, Parisi V, Femminella GD, Formisano R, Petraglia L, et al. (2013) Effects of exercise training on cardiovascular adrenergic system. Front Physiol 4: 348. [Crossref]
- Nagatomo T, Hosohata Y, Ohnuki T, Nakamura T, Hattori K, et al. (2001) Bopindolol: pharmacological basis and clinical implications. Cardiovasc Drug Rev 19: 9-24. [Crossref]
- Parvez B, Chopra N, Rowan S, Vaglio JC, Muhammad R, et al. (2012) A common ß1-adrenergic receptor polymorphism predicts favorable response to rate-control therapy in atrial fibrillation. J Am Coll Cardiol 59: 49-56.
- Schaller K, Mechau D, Scharmann HG, Weiss M, Baum M, et al. (1999) Increased training load and the beta-adrenergic-receptor system on human lymphocytes. J Appl Physiol (1985) 87: 317-324. [Crossref]
- Nemoto S, Hamawaki M, De Freitas G, Carabello BA (2002) Differential effects of the angiotensin-converting enzyme inhibitor lisinopril versus the beta-adrenergic receptor blocker atenolol on hemodynamics and left ventricular contractile function in experimental mitral regurgitation. J Am Coll Cardiol 40: 149-154.
- Behenke AR, Wilmore J (1974) Evaluation and regulation of body build and composition. Englewood Cliffs, N.J Prentile Hall, inc.
- American College of Sports Medicine (2014) ACSM's Guidelines for Exercise Testing and Prescription, 9th edition, Philadelphia, PA: Lippincott Williams & Wilkins 145-147 and 165-199.
- Karvonen MJ, Kentala E, Mustala O (1957) The effects of training on heart rate; a longitudinal study. Ann Med Exp Biol Fenn 35: 307-315. [Crossref]
- Staehelin M, Hertel C (1983) [3H] CGP-12177, a beta-adrenergic ligand suitable for measuring cell surface receptors. J Recept Res 3: 35-43. [Crossref]
- Staehelin M, Simons P, Jaeggi K, Wigger N (1983) CGP 12177: A hydrophilic /-adrenergic receptor ligand reveals high affinity binding of antagonist to intact cells. J Biol Chem 258: 3496-3502.
- Merlet P, Delforge J, Syrota A, Angevin E, Mazière B, et al. (1993) Positron Emission Tomography With 1C CGP-12177 to Assess -Adrenergic Receptor Concentration in Idiopathic Dilated Cardiomyopathy. Circulation 87: 1169-1178.
- Eynon N, Sagiv M, Amir O, Ben-Sira D, Goldhammer E, et al. (2008) The effect of long-term beta-adrenergic receptor blockade on the oxygen delivery and extraction relationship in patients with coronary artery disease. J Cardiopulm Rehabil Prev 28: 189-194.
- Mäki T, Kontula K, Härkönen M (1990) The beta-adrenergic system in man: physiological and pathophysiological response. Regulation of receptor density and functioning. Scand J Clin Lab Invest Suppl 201: 25-43. [Crossref]
- Brodde OE, Hillemann S, Kunde K, Vogelsang M, Zerkowski HR (1992) Receptor systems affecting force of contraction in the human heart and their alterations in chronic heart failure. J Heart Lung Transplant 11: S164-174.
- Brodde OE (2007) Beta-adrenoceptor blocker treatment and the cardiac beta-adrenoceptor-G-protein(s)-adenylyl cyclase system in chronic heart failure. Naunyn Schmiedebergs Arch Pharmacol 374: 361-372.
- Graafsma SJ, van Tits LJ, Willems PH, Hectors MP, Rodrigues de Miranda JF, et al. (1990) Beta 2-adrenoceptor up-regulation in relation to cAMP production in human lymphocytes after physical exercise. Br J Clin Pharmacol 30 Suppl 1: 142S-144S. [Crossref]
- Sagiv M, Ben-Sira D, Goldhammer E (2002) Beta-blockers, exercise, and the immune system in men with coronary artery disease. Med Sci Sports Exerc 34: 587-591. [Crossref]
- Kubo M, Cinader B (1990) Polymorphism of age-related changes in interleukin (IL) production: Differential changes of T helper subpopulations, synthesizing IL-2, IL-3 and IL-4. Eur J Immunol 20: 1289-1296.
- De Blasi A, Lipartiti M, Garattini S (1986) Beta-adrenergic receptor changes during tetatolol treatment in healthy volunteers: relevance for beta-blocking therapy. Am J Nephrol 6: 69-73.
- Molenaar P, Savarimuthu SM, Sarsero D, Chen L, Semmler AB, et al. (2007) Adrenaline elicits positive inotropic, lusitropic, and biochemical effects through beta 2 -adrenoceptors in human atrial myocardium from nonfailing and failing hearts, consistent with Gs coupling but not with Gi coupling. Naunyn Schmiedebergs Arch Pharmacol 375: 11-28.

