In this study, we have analyzed 200 people, 100 patients Migraine disease and 100 persons control group. The gene NOTCH3 analyzed in terms of genetic mutation made. In this study, people who have genetic mutation were targeted, with nervous disorders, Migraine disease. In fact, of all people with Migraine disease 100 patients Migraine disease had a genetic mutation in the gene NOTCH3 Migraine disease. Any genetic mutations in the target genes control group, did not show.
Genetic study, Migraine disease, Mutation The gene NOTCH3.
Today, neurological disorders, neuromuscular disorders are very important in creating. Including neurological disorders, including Migraine disease. Migraine disease is a neuromuscular disorder that commonly causes A progressive neurodegenerative disease with autosomal dominant inheritance, with the incidence in adulthood, the disease has three abnormal movements, cognitive disorders, psychological disorders known. Migraine disease is caused by genetic mutations, but also epigenetic factors are critical in inducing the disease.
Migraine is a primary headache disorder characterized by recurrent headaches that are moderate to severe [1]. Typically, the headaches affect one half of the head, are pulsating in nature, and last from two to 72 hours [1]. Associated symptoms may include nausea, vomiting, and sensitivity to light, sound, or smell [2]. The pain is generally made worse by physical activity [3]. Up to one-third of people have an aura: typically, a short period of visual disturbance which signals that the headache will soon occur [3]. Occasionally, an aura can occur with little or no headache following it [4].
Migraines are believed to be due to a mixture of environmental and genetic factors [5]. About two-thirds of cases run in families [6]. Changing hormone levels may also play a role, as migraines affect slightly more boys than girls before puberty and two to three times more women than men [7,8]. The risk of migraines usually decreases during pregnancy [7]. The underlying mechanisms are not fully known [9]. It is, however, believed to involve the nerves and blood vessels of the brain [6].
Migraines typically present with self-limited, recurrent severe headache associated with autonomic symptoms [6,17]. About 15-30% of people with migraines experience migraines with an aura [10,18] and those who have migraines with aura also frequently have migraines without aura [19] The severity of the pain, duration of the headache, and frequency of attacks are variable [6]. A migraine lasting longer than 72 hours is termed status migrainosus [20]. There are four possible phases to a migraine, although not all the phases are necessarily experienced [3]
• The prodrome, which occurs hours or days before the headache.
• The aura, which immediately precedes the headache.
• The pain phase, also known as headache phase.
• The postdrome, the effects experienced following the end of a migraine attack.
In this study,100 patients with Migraine disease and 100 persons control group were studied. Peripheral blood samples from patients and parents with written permission control was prepared. After separation of serum, using Real Time-PCR technique of tRNA molecules were collected. To isolate Neuroglial cells erythrocytes were precipitated from hydroxyethyl starch (HES) was used. At this stage, HES solution in ratio of 1to5with the peripheral blood of patients and controls were mixed. After 60 minutes of incubation at room temperature, the supernatant was removed and centrifuged for 14 min at 400 Gera. The cells ediment with PBS (phosphate buffered saline), pipetase and slowly soluble carbohydrate ratio of 1 to 2 on ficole (Ficol) was poured in the 480G was centrifuged for 34 minutes. Mono nuclear Neuroglial cells also are included, has a lower density than ficole and soon which they are based. The remaining erythrocytes has a molecular weight greater than fico le and deposited in test tubes.
The supernatant, which contained the mono nuclear cells was removed, and the 400 Gera was centrifuged for 12 minutes. Finally, the sediment cell, the antibody and Neuroglial cells was added after34 minutes incubation at 5 °C, the cell mixture was passed from pillar LSMACS. Then the cells were washed with PBS and attached to the column LSMACSS pam Stem cell culture medium containing the transcription gene NOTCH3 and were kept.
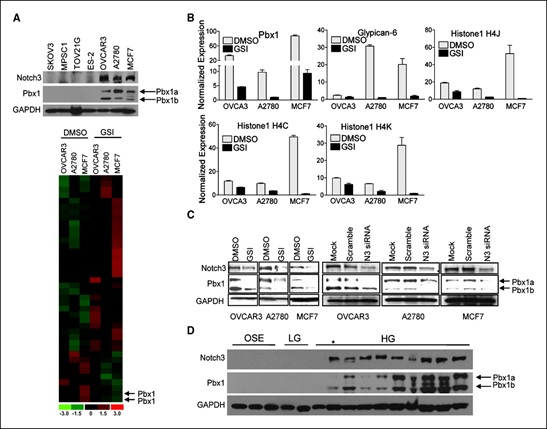
Figure 1. Schematic view of the pattern of gene NOTCH3 band formation in people with migraine.
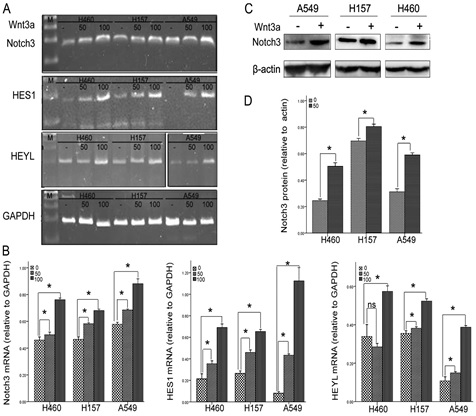
Figure 2. Schematic view of the pattern formed in the gene NOTCH3 band with diagram the expression of this gene in people with migraine
To determine the purity of Neuroglial cells are extracted, flow cytometry was used. For this purpose, approximately 4-5 × 103 Neuroglial cells were transfer red to1.5ml Eppendorf tube and then was centrifuged at 2000 rpm for 7minutes at time. Remove the supernatant culture medium and there maining sediment, 100μl of PBS buffer was added. After adding 5-10μl PE monoclonal anti body to the cell suspension for 60 min at 4°C, incubated and read immediately by flow cytometry. For example, rather than control anti body Neuroglial cells PE, IgG1 negative control solution was used.
Total mRNA extraction procedure includes:
1) 1ml solution spilled qiazol on cells, and slowly and carefully mixed and incubated at room temperature for 5 minutes. Then 200μl chloroform solution to target mix, then transfer the micro tubes were added and the shaker well was mixed for 15 seconds. The present mix for 4 minutes at room temperature and then incubated for 20 min at 4°Can was centrifuged at 13200 rpm era. Remove the upper phase product were transferred to a new microtube and to the one times the volume of cold ethanol was added. The resulting mixture for 24 hours at -20°C were incubated.
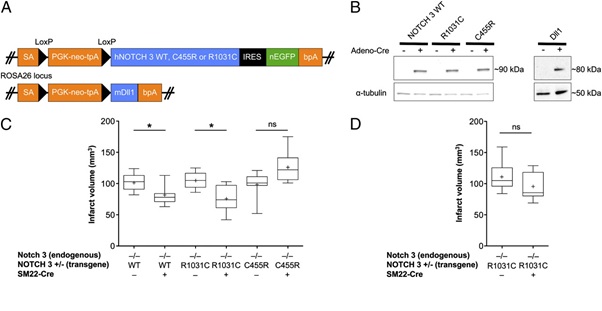
Figure 3. Schematic view of the pattern formed in the band NOTCH3 gene mutation in patients with migraine.
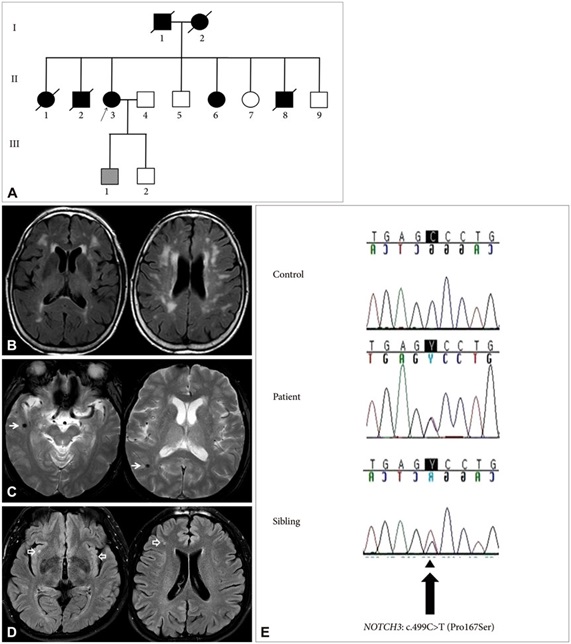
Figure 4. Schematic view of the pattern of inheritance in NOTCH3 gene mutation in patients with migraine and controls with brain scans
2)Then for 45min at 4°C was centrifuged at 12000 rpm era. Remove the supernatant and the white precipitate, 1ml of cold 75% ethanol was added to separate the sediment from micro tubes were vortex well. The resulting mixture for 20 min at 4°C by the time we were centrifuged 12000 rpm. Ethanol and the sediment was removed and placed at room temperature until completely dry deposition. The precipitate was dissolved in 20μl sterile water and at a later stage, the concentration of extracted mRNA was determined.
To assessment the quality of mi-RNAs, the RT-PCR technique was used. The cDNA synthesis in reverse transcription reaction(RT)kit (Fermentas K1622) and1μloligoprimers18(dT)was performed. Following the PCR reaction 2μM dNTP, 1μg cDNA, Fermentas PCR buffer1X, 0 / 75μM MgCl2, 1.25 U / μL Tag DNA at 95°C for4min, 95°C for 30s, annealing temperature 58°C for 30s, and72°C for30 seconds, 35 cycles were performed. Then 1.5% agarose gel, the PCR product was dumped in wells after electrophores is with ethidium bromide staining and color were evaluated.
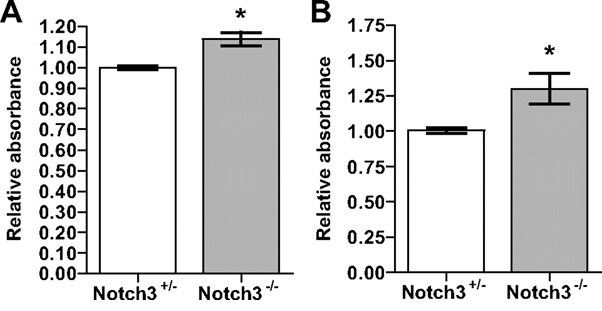
Figure 5. schematic diagram Relative absorbance NOTCH3 gene with mutation in patients with migraine.
According to the results of sequencing the genome of patients with Migraine disease and the genetic mutation NOTCH3 gene found that about 97% of patients with Migraine disease, they have this genetic mutation. Patients with Migraine disease, unusual and frightening images in the process of Migraine disease, experience. Lot epigenetic factors involved in Migraine disease. But the most prominent factor to induce Migraine disease, mutation is NOTCH3 gene. This gene can induce the birth and can also be induced in the adulthood.
Thanks to everyone who helped us in doing this project, very grateful. Patients and their families also accept patients who had very much for your cooperation in this study.
- Aminoff, Simon RP, Greenberg GA, Michael J (2009) Clinical neurology (7th Eds.,) New York, N.Y: Lange Medical Books/McGraw-Hill. Pp; 85-88.
- Headache Classification Subcommittee of the International Headache Society (2004) The International Classification of Headache Disorders" (2nd Eds.,). Cephalalgia 24: 9-160.
- Piane M, Lulli P, Farinelli I, Simeoni S, De Filippis S, et al. (2007) Genetics of migraine and pharmacogenomics: some considerations. J Headache Pain 8: 334-339. [crossref]
- Bartleson JD, Cutrer FM (2010) Migraine update. Diagnosis and treatment. Minn Med 93: 36-41. [crossref]
- Lay CL, Broner SW (2009) Migraine in women. Neurol Clin 27: 503-511. [crossref]
- Stovner LJ, Zwart JA, Hagen K, Terwindt GM, Pascual J (2006) Epidemiology of headache in Europe. Eur J Neurol 13: 333-345. [crossref]
- National Institute 2021 Copyright OAT. All rights reserv(2016) Retrieved 15 February 2016.
- Gilmore B, Michael M (2011) Treatment of acute migraine headache. Am Fam Physician 83: 271-280. [crossref]
- Diener HC, Charles A, Goadsby PJ, Holle D (2015) New therapeutic approaches for the prevention and treatment of migraine. Lancet Neurol 14: 1010-1022. [crossref]
- Armstrong C (2013) American Academy of, Neurology; American Headache, Society. "AAN/AHS update recommendations for migraine prevention in adults. American family physician 87: 584-595.
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, et al. (2012) Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380: 2163-2196.
- Bigal ME, Lipton RB (2008) The prognosis of migraine. Curr Opin Neurol 21: 301-308. [crossref]
- Buzzi MG, Cologno D, Formisano R, Rossi P (2005) Prodromes and the early phase of the migraine attack: therapeutic relevance. Functional neurology 20: 179-183.
- Rossi P, Ambrosini A, Buzzi MG (2005) Prodromes and predictors of migraine attack. Funct Neurol 20: 185-191. [crossref]
- Samuels, Ropper AH, Martin A (2009) Adams and Victor's principles of neurology (9th Eds,). New York: McGraw-Hill Medical. pp. Chapter 10.
- Tintinalli, Judith E (2010) Emergency Medicine: A Comprehensive Study Guide (Emergency Medicine (Tintinalli)). New York: McGraw-Hill Companies. Pp: 1116-1117.
- Kaniecki RG (2009) Basilar-type migraine. Curr Pain Headache Rep 13: 217-220. [crossref]
- Bose P, Goadsby PJ (2016) The migraine postdrome. Curr Opin Neurol 29: 299-301. [crossref]
- de Vries B, Frants RR, Ferrari MD, van den Maagdenberg AM (2009) Molecular genetics of migraine. Hum Genet 126: 115-132. [crossref]
- Montagna P (2008) Migraine genetics. Expert Rev Neurother 8: 1321-1330. [crossref]





