Abstract
Medical treatment using high-voltage electric potential (HELP) devices to generate an electric field (EF) is an alternative therapy commonly used in Japan. However, the underlying mechanisms of the potential health benefits are not fully understood. To address this issue, we performed plasma lipidomics using liquid chromatography in combination with tandem mass spectrometry (LC-MS/MS). 9-Hydroxyoctadecadienoic acid (HODE), 13-HODE, and 13-hydroperoxy-octadecadienoic acid (HpODE) levels were significantly upregulated after HELP (18 kV, 30 min) exposure. However, there was no effect on HODE-related diol- metabolites, epoxide- metabolites, ketone- metabolites, or prostaglandins (PGs). We further examined the effect of HELP exposure on plasma concentrations of mediators using enzyme-linked immunosorbent assay (ELISA)/enzyme immunoassay (EIA). Immunoreactive substance P (SP) and brain-derived neurotrophic factor (BDNF) levels were significantly upregulated after HELP exposure. Under these conditions, HELP exposure had no effect on immunoreactive levels of vasoactive intestinal peptide (VIP), bradykinin, calcitonin gene-related peptide (CGRP), or motilin. Our findings provide insight into the possible relationship between the pharmacological modulation of neuromediators and that of HODEs by EF exposure. They may also be important in the development of electroceuticals.
Key words
HODE, substance P, BDNF, TRPV1, electric field therapy
Abbreviations
α-MSH: alpha-melanocyte-stimulating hormone; AA: arachidonic acid; BDNF: brain-derived neurotrophic factor; CGRP: calcitonin gene-related peptide; COX: cycloxygenase; CYP4A: cytochrome P450 oxidase 4A; DiHOME: dihydroxy-octadecenoic acid; EF: electric field; EIA: enzyme immunoassay; ELISA: enzyme-linked immunosorbent assay; EpOME: epoxy-octadecenoic acid; GPR: G protein-coupled receptor; HELP: high-voltage electric potential; HETE: hydroxyeicosatetraenoic acid; HODE: hydroxyoctadecadienoic acid; HOTrE: hydroxyoctadecatrienoic acid; HpODE: hydroperoxy-octadecadienoic acid; LA: linoleic acid; LC-MS/MS: liquid chromatography in combination with tandem mass spectrometry; LOX: lipoxygenase; NGF: nerve growth factor; OEA: oleoylethanolamide; OxoODE: oxo-octadecadienoic acid; PG: prostaglamdin; 15-PGDH: 15-hydroxy prostaglandin dehydrogenase; PLA2: phospholipase A2; PLA2G2D: group IID secretory phospholipase A2; PPAR-γ: peroxisome proliferator-activated receptor-gamma: SP: substance P; TRPV1: transient receptor potential vanilloid 1; and VIP: vasoactive intestinal peptide.
Introduction
A therapeutic device designed to expose the human body to high-voltage electric potential (HELP) has been approved by the Ministry of Health, Labour and Welfare in Japan [1-9]. High-voltage electric field (EF) therapy is reported to be an effective treatment for stiff shoulders, constipation, insomnia, and headaches [1-9]. However, the mechanisms by which EF exposure induces its variety of health benefits are poorly understood. Key mediators, such as neuropeptide and endogenous metabolites, have been suggested as candidate molecules that represent the interface between symptoms and electroceutical target proteins [10-16]. Our previous attempts to find an EF exposure-induced biomarker using non-targeted plasma metabolomics led to the detection of changes in an endogenous lipid-derived signaling molecule oleoylethanolamide (OEA), and unsaturated fatty acids such as oleic acid, linoleic acid (LA), cis-11-eicosenoic acid, cis-11,14-eicosadienoic acid, cis-8,11,14-eicosatrienoic acid, cis-5,8,11,14,17-eicosapentaenoic acid, cis-4,7,10,13,16,19-docosahexaenoic acid, and arachidonic acid (AA) [15]. In particular, OEA activates the transient receptor potential vanilloid 1 (TRPV1) on perivascular sensory nerves [17]. In our previous study, we found that OEA induces marked upregulation in group IID secretory phospholipase A2 (PLA2G2D) expression in human subcutaneous cultured adipocytes [15]. Liberation of LA or AA in the PLA2 reaction is believed to represent the rate-limiting step of the cascade leading to the formation of bioactive lipid mediators. Thuren et al. reported that activation of PLA2-catalyzed hydrolysis was induced by EF [18]. Thus, we hypothesized that the increase in unsaturated fatty acids, such as LA and AA, after EF exposure may be affected to change in lipoxygenase (LOX)-derived metabolites in plasma. In this study, we investigated the levels of LA- and AA-derived lipid metabolites using LC-MS/MS in plasma samples obtained from healthy subjects before and after exposure to a single HELP stimulation. We report that the LA-derived lipid mediator 9-HODE and 13-HODE can be upregulated by HELP (18 kV, 30 min) exposure. Because 9-HODE treatment to cultured trigeminal ganglia neurons induces the release of sensory neuropeptide by the activation of TRPV1 [19], we also investigated the effect of HELP (18 kV, 30 min) exposure on several mediators.
Materials and methods
EF exposure
The system used for EF exposure has been previously described [8,9,15,16]. The EF system was equipped with a transformer, a seat, and two insulator-covered electrodes. One electrode was placed on a floor plate on which the subject’s feet were located, and the other was placed above the subject’s head. EF generated by the HELP apparatus (Healthtron PRO-18T, H9000, or HES-A30; Hakuju Institute for Health Science Co., Ltd., Tokyo, Japan) was uniformly created by transforming a 50-Hz alternating current at 18 kV. The safety of this system for human use was established by the Japanese government in 1963.
Subjects
Thirty-five healthy adults (12 males and 23 females; mean age, 46.3 ± 1.1 years; mean body mass index (BMI), 22.3 ± 0.5 kg/m2) participated in experiment 1 (exposure condition: 18 kV, 30 min). Ten healthy adults (3 males and 7 females; mean age, 46.8 ± 2.9 years; mean BMI, 22.5 ± 1.0 kg/m2) participated in experiment 2 (exposure condition: 18 kV, 30 min). Ten healthy adults (5 males and 5 females; mean age, 42.4 ± 2.8 years; mean BMI, 23.6 ± 1.0 kg/m2) participated in experiment 3 (exposure condition: 18 kV, 15 min). Ten healthy adults (5 males and 5 females; mean age, 42.5 ± 2.9 years; mean BMI, 21.4 ± 1.0 kg/m2) participated in experiment 4 (exposure condition: 9 kV, 15 min). Ten healthy adults (6 males and 4 females; mean age, 45.9 ± 2.9 years; mean BMI, 22.9 ± 1.0 kg/m2) participated in experiment 5 (exposure condition: 30 kV, 15 min). All experiments were performed in the morning and all participants signed an informed consent form after receiving verbal and written information about the study. All experiments were conducted in accordance with the Declaration of Helsinki and the study protocol was approved by the human ethics committee of Hakuju Institute for Health Science Co., Ltd. (Tokyo, Japan).
Plasma preparation
Blood samples were collected in vacutainer tubes coated with ethylenediaminetetraacetic acid (VP-NA070K; Terumo Corporation, Tokyo, Japan) and immediately centrifuged at 800 x g for 5 min to separate plasma from other cellular materials. Plasma was then transferred to a fresh Eppendorf tube and stored at -80°C until processing.
LC-MS/MS analysis
Lipid metabolites were measured as described previously [20]. LC-MS/MS analysis of lipid metabolites was performed using a API 4000 mass spectrometer (AB SCIEX, Foster City, CA, USA) equipped with the ACQUITY UPLC separation module (Waters Corporation, Milford, MA, USA). Chromotographic separation was performed at 40°C on a HSS T3 column (1.8 µm, 2.1 x 150 mm, Waters Corporation, Milford, MA, USA) under gradient conditions at a flow rate of 0.153 mL/min. The moblile phases consisted of 0.1% acetic acid/acetonitrile (7:3, v/v) and acetonitrile/2-propanol (1:1, v/v). The turbo ion spray interface was operated in the negative ion mode. Quantification was performed using Analyst 1.5.2 (AB SCIEX, Foster City, CA, USA). Multiple reaction monitoring (MRM) m/z transitions were 9-HODE=295 /171; 13-HODE=295/195; 9-HOTrE=293/171;13-HOTrE=293/195; 9,10-DiHOME=313/201; 12,13-DiHOME=313/183; 9(10)-EpOME=295/277;12(13)-EpOME=295/171; 9-HpODE=293/185; 13-HpODE=293/113; 9-OxoODE=293/185;13-OxoODE=293/113; 5-HETE=319/301; 12-HETE=319/301; 15-HETE=319/219; 20-HETE=319/301; PGD2=351/315; PGE2=351/271; PGF2α=353/309; 13,14-dihydro-15-keto-PGD2=351/333; 13,14-dihydro-15-keto-PGE2=351/333; and 13,14-dihydro-15-keto-PGF2α=353/182.
ELISA/EIA assay
Plasma immunoreactive levels of SP, VIP, bradykinin, CGRP, motilin, BDNF, and NGF were measured using a human SP EIA kit from Cayman Chemical (Ann Arbor, MI), human VIP and NGF ELISA kit from LifeSpan BioSciences (Seattle, WA), human bradykinin ELISA kit from Cloud-Clone (Houston, TX), human CGRP ELISA kit from Bertin Pharma (Montigny le Bretonneux, France), human motilin ELISA kit from Cusabio Biotech (Wuhan, Hubei, China), or human BDNF ELISA kit from Phoenix Pharmaceuticals (Belmont, CA).
Statistical analysis
Data were analyzed using Welch’s t-test. A probability (p) value < 0.05 was considered statistically significant.
Results
Effect of HELP exposure on lipid metabolites in plasma from healthy humans
We assessed LA-derived lipid metabolites in the plasma obtained from 35 healthy participants using LC-MS/MS. HELP (18 kV, 30 min) exposure showed significantly higher plasma concentrations of 9-HODE (2307 ± 142 vs. 1956 ± 136 pg/mL, p=0.004), 13-HODE (4419 ± 264 vs. 3762 ± 272 pg/mL, p=0.001), and 13-HpODE (486 ± 50 vs. 427 ± 47 pg/mL, p=0.016) than pre-exposure levels (Figure 1; Table 1). Under these conditions, HELP exposure had no effect on 9-hydroxyoctadecatrienoic acid (HOTrE); 13-HOTrE; 9,10-dihydroxy-octadecenoic acid (DiHOME); 12,13-DiHOME; 9(10)-epoxy-octadecenoic acid (EpOME); 12(13)-EpOME; 9-HpODE; 9-oxo-octadecadienoic acid (OxoODE); or 13-OxoODE (Figure 1; Table 1).
Metabolites |
Before (pg/mL) |
After (pg/mL) |
Ratio |
|
|
Mean ± SE |
Mean ± SE |
After/Before |
p value |
LA-derived (n = 35) |
Alcohols |
|
|
|
|
9-HODE |
1956 ± 136 |
2307 ± 142 |
1.18 |
0.004 ** |
13-HODE |
3762 ± 272 |
4419 ± 264 |
1.17 |
0.001 ** |
9-HOTrE |
80.4 ± 12.7 |
89.7 ± 14.3 |
1.12 |
0.221 |
13-HOTrE |
432 ± 66 |
428 ± 52 |
0.99 |
0.929 |
Diols |
|
|
|
|
9,10-DiHOME |
293 ± 30 |
326 ± 32 |
1.11 |
0.163 |
12,13-DiHOME |
1136 ± 140 |
1150 ± 76 |
1.01 |
0.898 |
Epoxides |
9(10)-EpOME |
514 ± 127 |
560 ± 69 |
1.09 |
0.627 |
12(13)-EpOME |
3829 ± 421 |
4338 ± 297 |
1.13 |
0.171 |
Hydroperoxides |
9-HpODE |
15.3 ± 2.0 |
16.9 ± 2.0 |
1.10 |
0.182 |
13-HpODE |
427 ± 47 |
486 ± 50 |
1.14 |
0.016 * |
Ketones |
9-OxoODE |
1131 ± 91 |
1080 ± 76 |
0.96 |
0.554 |
13-OxoODE |
756 ± 47 |
791 ± 55 |
1.05 |
0.351 |
AA-derived (n = 10) |
5-HETE |
530 ± 58 |
491 ± 25 |
0.93 |
0.351 |
12-HETE |
568 ± 139 |
433 ± 86 |
0.76 |
0.391 |
15-HETE |
268 ± 35 |
245 ± 16 |
0.91 |
0.445 |
20-HETE |
250 ± 54 |
259 ± 14 |
1.04 |
0.882 |
13,14-dihydro-15-keto-PGD2 |
4.60 ± 0.55 |
4.16 ± 0.39 |
0.90 |
0.272 |
13,14-dihydro-15-keto-PGE2 |
13.8 ± 2.4 |
12.8 ± 1.9 |
0.93 |
0.689 |
13,14-dihydro-15-keto-PGF2a |
13.0 ± 2.5 |
11.3 ± 1.9 |
0.87 |
0.521 |
PGD2 |
4.26 ± 1.32 |
4.26 ± 1.19 |
1.00 |
0.998 |
PGE2 |
6.18 ± 1.12 |
8.33 ± 1.11 |
1.35 |
0.220 |
PGF2a |
13.1 ± 2.4 |
10.2 ± 1.8 |
0.78 |
0.384 |
Table 1. Lipid metabolite profiles in human healthy plasma between before and after HELP (18 kV; 30 min) exposure.
* Indicates a significant difference (*p<0.05, ** p<0.01, t-test).
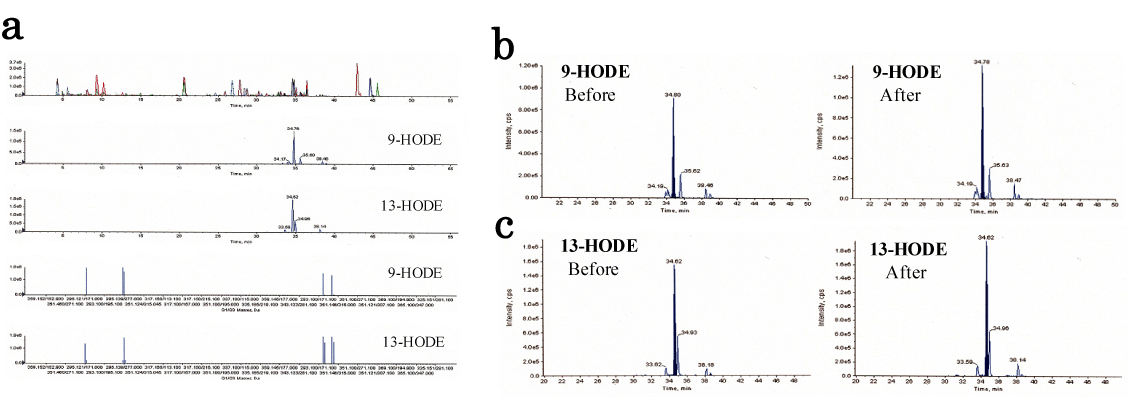
Figure 1. Lipidomic analysis of human healthy plasma between before and after HELP (18 kV) exposure for 30 min.
(a) Typical MS/MS spectrum for 9-HODE and 13-HODE from healthy human plasma sample. (b) Typical 9-HODE peak in healthy human plasma. (c) Typical 13-HODE peak in healthy human plasma.
To investigate whether other hydroxyl- metabolites were affected by HELP exposure, we tested the effect of HELP (18 kV, 30 min) exposure on AA-derived HETEs and PGs. As shown in Table 1, HELP exposure had no effect on 5-HETE; 12-HETE; 15-HETE; 20-HETE; 13,14-dihydro-15-keto-PGD2; 13,14-dihydro-15-keto-PGE2; 13,14-dihydro-15-keto-PGF2α;PGD2; PGE2; or PGF2α.
Effect of HELP exposure on immunoreactive SP levels in plasma from healthy humans at time points following EF
The results of EIA analysis of immunoreactive SP are shown in Figure 2a. Plasma imunoreactive SP concentrations significantly at the 30-min time point (A30) after HELP exposure when compared with pre-exposure levels (0 min, 1.83-fold, p=0.111; 30 min, 2.37-fold, p=0.032). The effect of HELP on immunoreactive SP levels was investigated using treatment for 15 min at 18 kV. The ratios of after/before were 1.43 (p=0.136), 1.72 (p=0.007), and 2.37 (p=0.003) for the 0-min time point (A0), 15-min time point (A15), and 45-min time point (A45), respectively (Figure 2b).
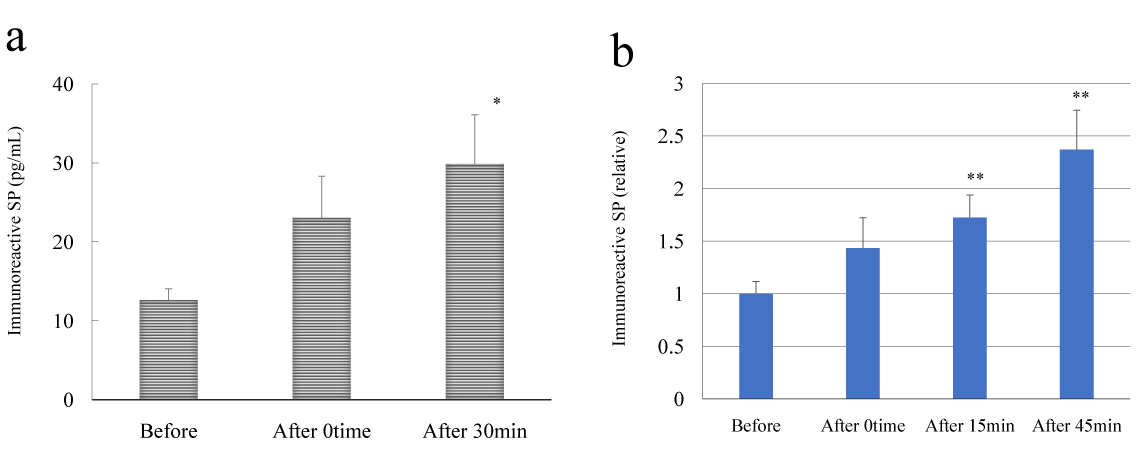
Figure 2. Effect of HELP exposure on immunoreactive SP levels in plasma from healthy humans at multiple time points.
(a) Effect of HELP (18 kV; 30 min) exposure on plasma immunoreactive SP levels at multiple time points. (b) Effect of HELP (18 kV, 15 min) exposure on plasma immunoreactive SP levels at multiple time points. Results are presented as mean ± SEM (n = 10). * p < 0.05 compared with before, ** p < 0.01 compared with before.
The nature of the immunoreactive SP responsible for EF strength was then investigated using treatment for 15 min. The ratios of immunoreactive SP levels after the 45-min time point (A45) / before were 1.04 (p=0.792) and 1.29 (p=0.011) for 9 and 30 kV, respectively.
Effect of HELP exposure on mediators in plasma from healthy humans at different time points following EF
Given that the release of SP is thought to contribute to the modulation of mediators [21,22], we evaluated the effect of 30 min of HELP exposure to 18 kV on neurotrophins and peptide hormones in plasma. As shown in Table 2, plasma immunoreactive BDNF concentrations significantly increased at the 30-min time point after HELP exposure compared with pre-exposure levels (1.39-fold, p=0.041). In contrast, plasma immunoreactive NGF concentrations significantly decreased immediately after HELP exposure compared with pre-exposure levels (0.88-fold, p=0.017). Under these conditions, HELP exposure did not affect the immunoreactive levels of VIP, bradykinin, CGRP, or motilin (Table 2).
Mediators |
Before
Mean ± SE |
0 min |
30 min |
After HELP
Mean ± SE |
After HELP
Mean ± SE |
n = 10 |
n = 10 |
n = 10 |
Peptide hormones |
|
|
|
VIP (pg/mL) |
221 ± 27 |
197 ± 27 |
227 ± 18 |
Bradykinin (pg/mL) |
11.0 ± 3.0 |
8.7 ± 2.1 |
7.1 ± 1.5 |
CGRP (pg/mL) |
51.2 ± 13.8 |
46.6 ± 10.4 |
38.4 ± 7.5 |
Motilin (pg/mL) |
28.3 ± 3.6 |
25.4 ± 1.7 |
27.4 ± 2.6 |
Neurotrophins |
|
|
|
BDNF (ng/mL) |
21.3 ± 1.8 |
23.9 ± 3.0 |
29.7 ± 4.4 * |
NGF (pg/mL) |
19.9 ± 3.6 |
17.5 ± 3.9 * |
20.1 ± 4.1 |
Table 2. Effect of HELP (18 kV, 30 min) exposure on mediators in plasma from healthy humans at multiple time points
*p < 0.05 compared with before.
The nature of the immunoreactive BDNF responsible for EF strength was then investigated using treatment for 15 min. The relative ratios of after 45-min time point (A45) / before were 1.39 (p=0.031) and 1.26 (p=0.006) for 9 and 30 kV, respectively.
Discussion
In this study, we showed that LA (18:2n-6)-derived hydroxy- (9-HODE and 13-HODE) and hydroperoxy- (13-HpODE) fatty acids in healthy human subjects are sensitive to acute EF exposure. However, AA (20:4n-6)-derived hydroxy-fatty acids (20-HETE, 15-HETE, 12-HETE, and 5-HETE) and AA-derived 13,14-dihydro-15-keto-PGs were not affected by EF exposure. 9-HODE and 13-HODE can be generated via 15-LOX in macrophages and vascular cells [23]. However, Upston et al. have shown that non-enzymatic oxidation of LA produces an approximately equal mixture of 9-HODE and 13-HODE [24]. Thus, EF exposure may generate, at least in part, 9-HODE and 13-HODE by non-enzymatic oxidation but not 5-LOX, 12-LOX, cytochrome P450 oxidase 4A (CYP4A), and cyclooxygenase (COX)/15-hydroxy prostaglandin dehydrogenase (15-PGDH). However, the detailed mechanisms of EF-induced changes in 9-HODE, 13-HODE, and 13-HpODE remain to be elucidated.
Our findings show that immunoreactive SP is upregulated by acute EF exposure. EF exposure did not appear to adversely alter physiological peptide hormone levels, at least those of VIP, bradykinin, CGRP, or motilin. The molecular mechanisms of changes in immunoreactive SP concentrations following EF exposure are complex and can be interpreted in several ways. Recently, a new member of the tachykinin family that displays high sequence identity with SP has been discovered [25]. To better understand the properties of immunoreactive SP, it is important to clarify the specificity of SP against the novel tachykinin peptide family including hemokinin 1. Further studies may elucidate the identity of immunoreactive SP. TRPV1 is a nonselective cation channel present on sensory neurons that is activated by heat (> 43°C), protons, capsaicin, and endovanilloids [26-28]. OEA, an endovanilloid, has been suggested to function as an endogenous agonist of TRPV1 [17,29]. Kendall et al. recently reported that OEA and LA-derived hydroxy fatty acids, such as 9- and 13-HODE, were present in human skin at high concentrations [30]. Neuropeptides, such as SP and CGRP, are present in human skin [31]. Interestingly, Patwardhan et al. reported that 9-HODE and 13-HODE as endogenous ligands for TRPV1 were formed in animal skin biopsies after exposure to noxious heat at a temperature range of 40-55°C [19]. Of note is that application of 9-HODE to cultured trigeminal ganglia neurons stimulates the release of neuropeptide such as CGRP [19]. Nathan et al. reported that TRPV1-mediated SP release from primary sensory neurons [32]. Moreover, Miranda-Morales et al. have recently reported that axon reflexes evoked by TRPV1 activation are mediated by tetrodotoxin-resistant voltage-gated Na+ channels in intestinal afferent nerves [33]. There is also evidence that electrical stimulation has a stimulating effect on release of SP from peripheral nerve terminals in the skin [34]. Thus, several endogenous ligands for TRPV1 may exert effects via SP release induced by the axon reflex at nerve terminals of peripheral sensory neurons in human intestine or skin [35]. Considerable evidence on the prevention of swallowing disorders has been obtained from studies of improvement of the swallowing reflex by capsaicin administration [36]. Ebihara et al. reported that administration of capsaicin improved the swallowing reflex by increasing SP levels [37,38]. In future, it will be important to assess the alleviative effect on dysphagia in clinical trial using EF exposure.
SP, LA, 13-HODE, and 13-HpODE induces endothelial-mediated vasorelaxation in the coronary artery of the pig [39,40]. Interestingly, OEA also causes endothelium-dependent vasorelaxation in the rat small mesenteric artery [41]. OEA activates on perivascular sensory nerves and induces neuropeptide release [41]. A similar vasodilation was reported by Tochio et al. in a study examining the effect of EF exposure on the rat small mesenteric artery [42]. Thus, it is conceivable that the increase of 9-HODE, 13-HODE, 13-HpODE, OEA, and immunoreactive SP levels in plasma is, at least in part, responsible for the improvement observed in patients with stiff shoulders who undergo EF treatment [7].
Capsaicin is used for chronic pain relief as a defunctionalization inducer of nociceptor [27,28,43]. For example, a capsaicin dermal patch is available for the treatment of peripheral neuropathic pain [44]. Our previous study has shown that acute EF (18 kV, 30 min) exposure induces an increase in plasma OEA levels [15]. An experimental pretest-posttest design study by Shinba et al. showed that repetitive stimulation with EF exposure reduced the visual analog scale for pain in chronic pain with no obvious underlying disease [8]. Although repetitive EF treatment was not performed in this study, EF exposure may alleviate pain, at least in part, via desensitization of TRPV1 by 9-HODE, 13-HODE, or OEA. However, 9-HODE and 13-HODE also activates peroxisome proliferator-activated receptor-gamma (PPAR-γ) [45], raising the possibility that these receptors also serve as targets for 9-HODE/13-HODE during EF exposure. Further studies are in progress.
The results of this study also indicate that acute EF exposure affects plasma BDNF levels in healthy human subjects. Considerable evidence for the regulation of BDNF by alpha-melanocyte-stimulating hormone (α-MSH) has been obtained from the study of BDNF expression [46]. Nicholson et al. reported that BDNF release was induced by stimulation of the melanocortin-4 receptor [47]. We have reported that EF exposure induces an increase of the nonselective melanocortin receptor agonist α-MSH levels in plasma [16]. It is thus reasonable to speculate that EF exposure activates BDNF release/secretion or production through the upregulation of α-MSH. However, it is unclear at present whether changes in BDNF levels can be attributed to neurons, astrocytes, microglia, mast cells, fibroblasts, leukocytes, platelets, or keratinocytes. Further studies are needed to identify the BDNF signaling pathways induced by EF exposure. Notably, intact BDNF in peripheral circulation can cross the blood-brain barrier via a high-capacity, saturable transport system [48]. Positive correlations between blood BDNF and hippocampal BDNF levels have been observed in rats and pigs [49]. Neurotrophic activities in the hippocampus have been suggested to play a key role in spatial learning and memory function [50,51]. Interestingly, Yanamoto et al. have reported that EF exposure (5 h/day for 3 weeks) induces an increase of hippocampal BDNF levels in mice and an improvement of Morris water maze tasks in infarct lesions of mice [52]. In contrast, Campolongo et al. reported that post-training administration of OEA in rats enhances memory consolidation in a Morris water maze performance [53]. Thus, it is reasonable to speculate that EF exposure facilitates spatial learning and memory function via upregulation of OEA and BDNF. A recent study has shown negative correlations between plasma BDNF levels and objective evaluation of tinnitus severity [54]. In future, it will be of interest to evaluate the possible effect of EF exposure on tinnitus.
In conclusion, acute EF exposure exerted marked effects on plasma 9-HODE, 13-HODE, SP, and BDNF levels in healthy subjects. Our findings provide insight into the molecular mechanisms of health benefits induced by the HELP device (PRO-18T) and may also be important in the development of therapies for dysphagia, chronic pain, mild cognitive impairment, and tinnitus.
Competing interests
YN-Y, HH and AH are employed by Hakuju Institute for Health Science Co., Ltd., FN is employed by CMIC Pharma Science Co., Ltd., and MS is employed by Acel Inc.. All other authors have no competing interests.
Authors’ contributions
YN-Y designed and supervised the research, and wrote the manuscript. FN performed LC-MS/MS. YN-Y, MS, HH, and AH performed the EF exposure and biochemical experiments. All authors have read and approved the final version of the manuscript.
References
- Hara H (1961) On the effect of AC. electrostatic high voltage potential load upon the blood-electrolytes (in Japanese). Niigata Medical J 75: 265-273.
- Ito F, Furuya K (1981) The effect of high voltage alternating current upon a human body the change of blood pressure, endocrine system and serum lipids (in Japaneses). J Jpn Sci Balneol Climatol Phys Med 45: 6-17.
- Nawarat S, Iomsai K, Jantanam P, Kauengtip Y (1999) Effects of electrical Healthtron on curing of non-communicable diseases : Case study of Banlad hospital Petchaburi province (in Thai). Region 4 Medical J 18: 139-149.
- Ito F (2000) The role of electric field therapeutic device (Healthtron) in the therapy of acute low back pain (in Japanese). J Jpn Sci Balneol Climatol Phys Med 63: 127 -137.
- Siripanichgon K, Otrakul A, Suparp J, Sirikulchayanonta C, Charupoonphol P (2000) Clinical observation of Healthtron therapy (in Thai). J Public Health (Bangkok) 30: 19-29.
- Sirikulchayanonta C, Siripanichgon K, Otrakul A, Suparp J, Charupoonphol P (2001) The effect of Healthtron on serum lipid levels among the middle-aged: Preliminary report. J Public Health (Bangkok) 31: 63-70.
- Ito F, Ohsaki K, Takahashi K, Hara H (2005) The effects of electric field therapeutic device (Healthtron) on the stiffness in the neck and shoulder area – changes in subjective symptoms, blood circulation and the autonomic nervous system ( in Japaneses). J Jpn Sci Balneol Climatol Phys Med 68: 110-121.
- Shinba T, Takahashi K, Kanetaka S, Nedachi T, Yamaneki M, et al. (2012) A pilot study on electric field therapy for chronic pain with no obvious underlying diseases (in Japanese). Soc Integrative Med Jpn 5: 68-72.
- Mitani Y, Matsugi A2021 Copyright OAT. All rights reservffect of exposure to a high-voltage alternating current electric field on muscle extensibility. J Jpn Sci Balneol Climatol Phys Med 78: 244-252.
- Nakagawa-Yagi Y, Ogane N, Inoki Y, Kitoh N (1996) The endogenous estrogen metabolite 2-methoxyestradiol induces apoptotic neuronal cell death in vitro. Life Sci 58: 1461-1467. [Crossref]
- Kanasaki K, Palmsten K, Sugimoto H, Ahmad S, Hamano Y, et al. (2008) Deficiency in catechol-O-methyltransferase and 2-methoxyoestradiol is associated with pre-eclampsia. Nature 453: 1117-1121. [Crossref]
- Chowdhury R, Yeoh KK, Tian YM, Hillringhaus L, Bagg EA, et al. (2011) The oncometabolite 2-hydroxyglutarate inhibits histone lysine demethylases. EMBO Rep 12: 463-469. [Crossref]
- Patti GJ, Yanes O, Shriver LP, Courade JP, Tautenhahn R, et al. (2012) Metabolomics implicates altered sphingolipids in chronic pain of neuropathic origin. Nat Chem Biol 8: 232-234. [Crossref]
- Piomelli D, Sasso O (2014) Peripheral gating of pain signals by endogenous lipid mediators. Nat Neurosci 17: 164-174. [Crossref]
- Nakagawa-Yagi Y, Hara H, Fujimori T, Yamaguchi T, Midorikawa A, et al. (2014) Non-targeted human plasma metabolomics reveals the changes in oleoylethanolamide, a lipid-derived signaling molecule, by acute exposure of electric field. Integr Mol Med 1: 29-37.
- Nakagawa-Yagi Y, Hara H, Yoshida Y, Midorikawa A, Hara A (2015) Discovery of a novel effect of electric field exposure on human plasma beta-endorphin and interleukin-12 levels: Insight into mechanisms of pain alleviation and defense against infection by electric field therapy. Integr Mol Med 2: 200-204.
- Movahed P, Jönsson BA, Birnir B, Wingstrand JA, Jørgensen TD, et al. (2005) Endogenous unsaturated C18 N-acylethanolamines are vanilloid receptor (TRPV1) agonists. J Biol Chem 280: 38496-38504. [Crossref]
- Thuren T, Tulkki AP, Virtanen JA, Kinnunen PK (1987) Triggering of the activity of phospholipase A2 by an electric field. Biochemistry 26: 4907-4910. [Crossref]
- Patwardhan AM, Akopian AN, Ruparel NB, Diogenes A, Weintraub ST, et al. (2010) Heat generates oxidized linoleic acid metabolites that activate TRPV1 and produce pain in rodents. J Clin Invest 120: 1617-1626. [Crossref]
- Kondo K, Morino K, Nishio Y, Kondo M, Nakao K, et al. (2014) A fish-based diet intervention improves endothelial function in postmenopausal women with type 2 diabetes mellitus: A randomized crossover trial. Metab Clin Exp 63: 930-940. [Crossref]
- Burbach GJ, Kim KH, Zivony AS, Kim A, Aranda J, et al. (2001) The neurosensory tachykinins substance P and neurokinin A directly induce keratinocyte nerve growth factor. J Invest Dermatol 117: 1075-1082. [Crossref]
- Julius D, Basbaum AI (2001) Molecular mechanisms of nociception. Nature 413: 203-210. [Crossref]
- Vangaveti V, Baune BT, Kennedy RL (2010) Hydroxyoctadecadienoic acids: novel regulators of macrophage differentiation and atherogenesis. Ther Adv Endocrinol Metab 1: 51-60. [Crossref]
- Upston JM, Neuzil J, Wirtting PK, Alleva R, Stocker R (1997) Oxidation of free fatty acids in low density lipoprotein by 15-lipoxygenase stimulates nonenzymic, ?-tocopherol-mediated peroxidation of choleseryl esters. J Biol Chem 272: 30067-30074.
- Bellucci F, Carini F, Catalani C, Cucchi P, Lecci A, et al. (2002) Pharmacological profile of the novel mammalian tachykinin, hemokinin 1. Br J Pharmacol 135: 266-274. [Crossref]
- Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, et al. (1998) The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21: 531-543. [Crossref]
- Vriens J, Appendino G, Nilius B (2009) Pharmacology of vanilloid transient receptor potential cation channels. Mol Pharmacol 75: 1262-1279. [Crossref]
- Szolcsányi J, Sándor Z (2012) Multisteric TRPV1 nocisensor: a target for analgesics. Trends Pharmacol Sci 33: 646-655. [Crossref]
- Ahern GP1 (2003) Activation of TRPV1 by the satiety factor oleoylethanolamide. J Biol Chem 278: 30429-30434. [Crossref]
- Kendall AC, Pilkington SM, Massey KA, Sassano G4, Rhodes LE2, et al. (2015) Distribution of bioactive lipid mediators in human skin. J Invest Dermatol 135: 1510-1520. [Crossref]
- Slominski A, Wortsman J (2000) Neuroendocrinology of the skin. Endocr Rev 21: 457-487. [Crossref]
- Nathan JD, Patel AA, Mcvey DC, Thomas JE, Prpic V, et al. (2001) Capsaicin vanilloid receptor-1 mediates substance P release in experimental pancreatitis. Am J Physiol Gastrointest Liver Physiol 281: G1322-G1328. [Crossref]
- Miranda-Morales M, Ochoa-Cortes F, Stern E, Lomax AE, Vanner S (2010) Axon reflexes evoked by transient receptor potential vanilloid 1 activation are mediated by tetrodotoxin-resistant voltage-gated Na+ channels in intestinal afferent nerves. J Pharmacol Exp Ther 334: 566-575. [Crossref]
- White DM, Helme RD (1985) Release of substance P from peripheral nerve terminals following electrical stimulation of the sciatic nerve. Brain Res 336: 27-31. [Crossref]
- Yaprak M (2008) The axon reflex. Neuroanatomy 7: 17-19.
- Sasaki H, Yamaya M, Ohrui T, Kubo H, Ebihara M, et al. (2002) Characteristics of respiratory diseases in older people. JMAJ 45: 231-236.
- Ebihara T, Sekizawa K, Nakazawa H, Sasaki H (1993) Capsaicin and swallowing reflex. Lancet 341: 432. [Crossref]
- Ebihara T, Takahashi H, Ebihara S, Okazaki T, Sasaki T, et al. (2005) Capsaicin troche for swallowing dysfunction in older people. J Am Geriatr Soc 53: 824-828. [Crossref]
- Kuroiwa M, Aoki H, Kobayashi S, Nishimura J, Kanaide H (1995) Mechanism of endothelium-dependent relaxation induced by substance P in the coronary artery of the pig. Br J Pharmacol 116: 2040-2047. [Crossref]
- Pomposiello SI, Alva M, Wilde DW, Carretero OA (1998) Linoleic acid induces relaxation and hyperpolarization of the pig coronary artery. Hypertension 31: 615-620. [Crossref]
- AlSuleimani YM, Hiley CR (2013) Mechanisms of vasorelaxation induced by oleoylethanolamide in the rat small mesenteric artery. Eur J Pharmacol 702: 1-11. [Crossref]
- Tochio K, Harakawa S, Nedachi T, Hori T, Takahashi K, et al (2008) Application of imaging method for measuring microcirculation to evaluate effects induced by exposure to electric field in peripheral microcirculation (in Japanese). Jpn J Medical Instrumentation 78: 125-130.
- Anand P, Bley K (2011) Topical capsaicin for pain management: therapeutic potential and mechanisms of action of the new high-concentration capsaicin 8% patch. Br J Anaesth 107: 490-502. [Crossref]
- Noto C, Pappagallo M, Szallasi A (2009) NGX-4010, a high-concentration capsaicin dermal patch for lasting relief of peripheral neuropathic pain. Curr Opin Investig Drugs 10: 702-710. [Crossref]
- Itoh T, Fairall L, Amin K, Inaba Y, Szanto A, et al. (2008) Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol 15: 924-931. [Crossref]
- Caruso C, Carniglia L, Durand D, Gonzalez PV, Scimonelli TN, et al. (2012) Melanocortin 4 receptor activation induces brain-derived neurotrophic factor expression in rat astrocytes through cyclic AMP – protein kinase A pathway. Mol Cell Endocrinol 348: 47-54. [Crossref]
- Nicholson JR, Peter JC, Lecourt AC, Barde YA, Hofbauer KG (2007) Melanocortin-4 receptor activation stimulates hypothalamic brain-derived neurotrophic factor release to regulate food intake, body temperature and cardiovascular function. J Neuroendocrinol 19: 974-982. [Crossref]
- Pan W, Banks WA, Fasold MB, Bluth J, Kastin AJ (1998) Transport of brain-derived neurotrophic factor across the blood-brain barrier. Neuropharmacology 37: 1553-1561. [Crossref]
- Klein AB, Williamson R, Santini MA, Clemmensen C, Ettrup A, et al. (2011) Blood BDNF concentrations reflect brain-tissue BDNF levels across species. Int J Neuropsychopharmacol 14: 347-353. [Crossref]
- Nakagawa Y, Nakamura S, Kaśe Y, Noguchi T, Ishihara T (1987) Colchicine lesions in the rat hippocampus mimic the alterations of several markers in Alzheimer's disease. Brain Res 408: 57-64. [Crossref]
- Nakagawa Y, Ishihara T (1988) Enhancement of neurotrophic activity in cholinergic cells by hippocampal extract prepared from colchicine-lesioned rats. Brain Res 439: 11-18. [Crossref]
- Yanamoto H, Miyamoto S, Nakajo Y, Nakano Y, Hori T, et al. (2008) Repeated application of an electric field increases BDNF in the brain, enhances spatial learning, and induces infarct tolerance. Brain Res 1212: 79-88.
- Campolongo P, Roozendaal B, Trezza V, Cuomo V, Astarita G, et al. (2009) Fat-induced satiety factor oleoylethanolamide enhances memory consolidation. Proc Natl Acad Sci U S A 106: 8027-8031. [Crossref]
- Goto F Saruta J, Kanzaki S, To M, Tsutsumi T, et al. (2012) Various levels of plasma brain-derived neurotrophic factor in patients with tinnitus. Neurosci Lett 510: 73-77. [Crossref]


