Abstract
A new hysteroscopic technique (ReLARC®) for the insertion of long-term contraceptive devices is described. The procedure is conducted under direct visualization; the method is simple, safe and rapid, and is suitable for application in the operating room or the office. The method is a reversible alternative to more complex insertion techniques for hysteroscopic intratubal contraception. It is the first hysteroscopically inserted system suitable for long-term intrauterine contraception. The copper-based contraceptive devices have a duration of action up to 10 years, or longer, and are immediately effective, contrary to existing hysteroscopic methods, and have a track record of high tolerance and acceptability. Some examples are briefly described to illustrate the new method and use of the device.
Key words
IUD, LARC, hysteroscopy, reversible contraception
Introduction
Long-acting reversible contraceptive methods (LARC) are frequently advocated for use in women, including nulliparous women and adolescents [1]. However, current conventional designed IUDs have drawbacks (i.e., pain, abnormal bleeding, expulsion) as most are too large for a vast number of women and may account for the high discontinuation rates seen with many conventional T-shaped IUDs, approaching 40 to 50% of users by year 5 [2]. As the devices have no frame, they will fit in uterine cavities of various sizes and shapes. Their unique method of anchoring, which allows for the complete elimination of a crossarm of any type, assures long term retention, and importantly allows for passage of the device through the working channel of a special hysteroscope. The ability to visualize the uterus prior to insertion, to assess uterine compatibility and the assurance of proper device placement are important aspects that visual insertion, such as with hysteroscopy, would allow.
ReLARC® (Reversible Long Acting Reproductive Control, Contrel Europe, Ghent, Belgium) was developed in order to maximize patient and physician access to this new frameless technology to allow the procedure to be carried out under direct visualization to maximize optimal insertion. The hysteroscopic technique could also be advantageous in the presence of anomalies of the uterus or uterine cavity, or can easily be used in combination with many frequently performed hysteroscopic surgeries/procedures (i.e., following removal of malpositioned, displaced, or embedded IUDs, etc).
ReLARC was initially developed for long-term non-hormonal contraception, as a reversible alternative to irreversible hysteroscopic sterilization (e.g., Essure®) or surgical sterilization procedures. A hormonal version, consisting of a fibrous drug delivery system, releasing 14 µg/d of levonorgestrel, and of a size suitable for hysteroscopic insertion is also currently in late stage development. A hormone releasing system not only will address contraception but its secondary pharmacologic actions would allow it to be used in conjunction with other hysteroscopic procedures such as fibroid and polyp resection and possibly endometrial ablation, etc). Having the ability to select either a copper or levonorgestrel (LNG) delivery system of similar design for hysteroscopic insertion would provide maximal contraceptive flexibility to women and high continuation use rates to the optimal design characteristics of the ReLARC systems.
Office or day-clinic hysterosocopy procedures have had an increasing impact on the practice of gynecology as it allows for various surgical procedures to be performed in a minimally invasive manner with reduced patient risk, shorter recovery periods and improved clinical benefit. To date, most of its uses have been limited to modified surgical procedures. We describe a new hysteroscopic technique, designed to provide long-term continual intrauterine contraception, rivaling that achievable with conventional IUD and sterilization techniques but with the major advantage of being fully reversible. The procedure is intended to expand the use of hysteroscopy for minimally invasive procedures while providing women with a convenient and comfortable alternative in case of problems with current IUDs, or with a reversible alternative to surgical sterilization.
Description of the method and insertion technique
ReLARC consists of a length of non-biodegradable 0-size, blue monofilament polypropylene suture with copper cylinders threaded on it. The cylinders are freely moving allowing the device to flex and shift in vivo. The upper and lower tubes are crimped onto the thread to keep them in place. Several types are available depending on the copper load, 240 mg, 350 mg and 420 mg, respectively, with a copper surface area of 250 mm2, 300 mm2, and 380 mm2, respectively. The lifespan varies from 3 to up to 10 years, possibly longer.
The levonorgestrel-releasing system is similar as it uses the same anchoring technique but instead of copper tubes a 3.3 cm long and thin fibrous delivery system, 1.2 mm in diameter, releasing approximately 14 μg of LNG per day, is fixed to the anchoring thread by means of a stainless steel clip 1 cm from the anchoring knot.
The upper extremity of the thread, in both the copper and LNG versions, ends in a knot (Figure 1) which is implanted into the myometrium of the uterine fundus using a specially designed inserter which is inserted through the working channel of an office hysteroscope, thereby permanently securing the device in the uterine cavity (Figure 2). The anchor is visible on ultrasound allowing precise location of the anchor at insertion and follow-up (Figure 3).
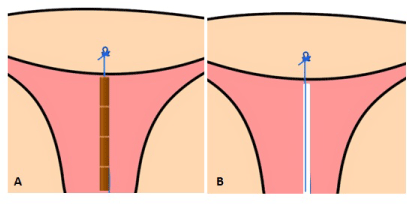
Figure 1. Illustration of the anchoring concept: the polypropylene 0-gauge suture with anchoring knot inserted in de uterine fundus. The anchoring system acts as a means of suspension for A) copper elements, or B) a bioactive substance such as a drug delivery system for the sustained release of LNG.
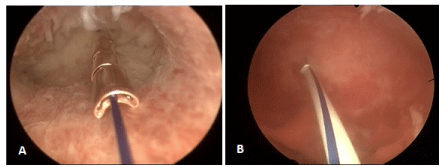
Figure 2. A) Hysteroscopic view of ReLARC® Copper anchored in the uterine fundus and B) ReLARC® LNG. Note the reduced space occupied by the devices in the uterine cavity contributing to their optimal tolerance and acceptability which is required for a long-term use.
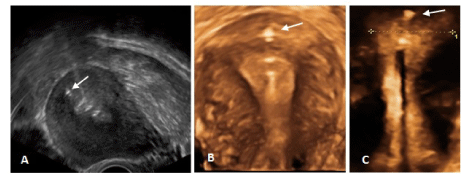
Figure 3. A) 2D ultrasound of anchor (arrow), showing correct position of the anchor in the uterine fundus; B) 3D ultrasound of ReLARC® copper anchored in the fundus (arrow); C) 3D ultrasound of ReLARC® LNG with visualized anchor (arrow). Figures B and C also shows the optimal harmony of ReLARC® in narrow uterine cavities.
The inserter has to be used with a hysteroscope with working channel of 3 mm. The depth of insertion is visually controlled by direct hysteroscopic vision as well as through the use of vaginal or abdominal ultrasound assuring the precise placement of ReLARC as shown in Figures 2 and 3. Although the copper devices are small, they have an efficacy greater than that seen with other contraceptive procedures and intrauterine devices [2]. ReLARC differs from conventional T-shaped IUDs as it uses small copper cylinders not only to assure device flexibility in utero but also to allow for copper ions to be released from the outside as well as from the inside of the copper tubes accounting for its high efficacy [3].
The new hysteroscopic method, is derived from long-term intrauterine contraceptive device (IUD) research in an attempt to improve the acceptability of intrauterine contraception, the two major contributors to a low continuation rate of IUDs being spontaneous expulsion and patient-requested removal for bleeding and/or pain [2]. ReLARC is the first reversible hysteroscopically inserted intrauterine contraceptive method that can rival irreversible sterilization methods with respect to efficacy and tolerability. One of the important advantages of the method is that the uterine cavity can be accessed much easier and simpler than the fallopian tubes. The uterus itself can be explored thoroughly by hysteroscopy before the intrauterine contraceptive method is applied and ReLARC can always be placed visually, in contrast with intratubal devices.
Case reports
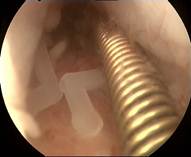
Five case reports are summarized in Table 1 as examples, illustrating the position of the ReLARC and its compatibility with the uterine cavity. Insertions were done under sedation or total intravenous anesthesia (TIVA) for administering short-acting, potent hypnotic (Disoprivan®) and analgesic (Ultiva®) drugs, and a laryngeal mask, or by using paracervical block anesthesia.
Table 1. Summary of cases of ReLARC® insertion, described as examples, including the hysteroscopic views before (shown on the left) and after anchoring of ReLARC® in the fundus of the uterus (shown on the right right).
|
Case 1 is a 28-year old nulligravida with dislocated MLCu375 IUD. On 2D ultrasound examination, the IUD was displaced in the uterine cervix which was found to be compressed in the cervical canal on hysteroscopic examination (left). ReLARC was inserted in the middle of the fundus (right). |
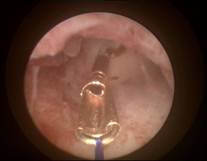
|
|
|
|
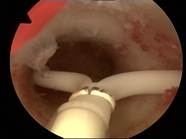
|
Case 2 is a young 22-year old nulliparous women with both arms of Jaydess®/Skyla® embedded in the cervix. The uterine cavity is empty. The uterine cavity width is ~24 mm. The LNG-IUS was removed and ReLARC was anchored in the middle of the fundus (right). |
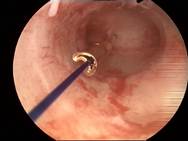
|
|
|
|
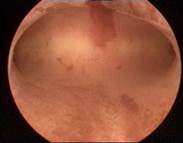
|
Case 3 is a G3P1, 36-year old woman, 18 weeks postpartum with wide uterine cavity of ~40 mm (left). She lost a T-shaped IUD. The woman requested long-term intrauterine contraception and opted for ReLARC with a lifespan of 10 years which was inserted in the midline of the uterine fundus (right). |
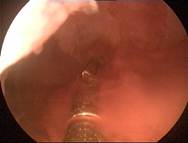
|
|
|
|
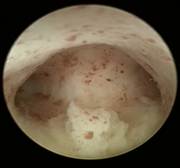
|
Case 4 is a young, 18-year-old nulligravida with uterus arcuatus and wish to be fitted with ReLARC. The uterine cavity was narrow (21 mm) (left). ReLARC was inserted in the middle of the uterine fundus (right). |
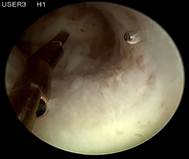
|
|
|
|
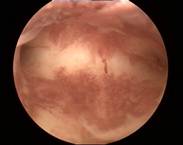
|
Case 5 is a 38-year old G1P1 who had Mirena inserted 2 years earlier and had amenorrhea for one year and then developed continuous spotting. Mirena was found with the stem partly in the vagina. The cavity was ~24 mm wide. ReLARC (10 years) was inserted in the midline (right). |
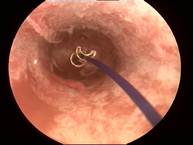
|
Conclusion
ReLARC is simple and safe to insert in the office or clinical setting, taking only approximately 15 minutes once proficiency is acquired. The echogenic anchor with biocompatible stainless steel marker allows the clinician the ability to check placement and assure the proper position of the anchor with ultrasound. ReLARC can easily be removed by pulling the tail with minimal patient discomfort, causing no damage to the uterus, and resulting in immediate return of fertility [4].
Prior to insertion, any uterine abnormalities, presence of fibroids, including adequacy of the cavity to accommodate ReLARC, can be diagnosed and treated. Hysteroscopic insertion allows for direct visualization, and controlled and precise device insertion, even when anomalies or uterine deformities are present, and can also be placed following an intervention.
ReLARC offers hysteroscopists the opportunity to provide their patients a safe, simple and long-acting method that is non-encombrant and is highly suitable as an alternative to sterilization that has the added benefit of reversibility.
Declaration of interest
Thomas Hasskamp, MD: none declared. Dirk Wildemeersch, MD, PhD, is the developer of intrauterine devices and systems. He has also been involved in the development and optimization of new, innovative, drug delivery systems for use in the uterus. He did not receive any financial compensation of any kind. Currently he acts as an advisor in devising new concepts in controlled release for contraception and gynecological treatment.
References:
- American College of Obstetricians and Gynecologists. Committee opinion number 539: Adolescents and long-acting reversible contraception: implants and intrauterine devices. 2012. Available from: https://www.acog.org/Resources_And_Publications/Committee_Opinions/Committee_on_Adolescent_Health_Care/Adolescents_and_Long-Acting_Reversible_Contraception. Accessed May 29, 2014.
- Wildemeersch D, Goldstuck ND, Hasskamp T (2016) Intrauterine systems: a frameless future? Expert Opin Drug Deliv 13: 911-918. [Crossref]
- Wildemeersch D, Sab2021 Copyright OAT. All rights reserv P, et al. (2014) Assessment of copper corrosion from frameless copper IUDs after long-term in utero residence. Contraception 90: 454–459. [Crossref]
- Delbarge W, Bátár I, Bafort M, Bonnivert J, Colmant C, et al. (2002) Return to fertility in nulliparous and parous women after removal of the GyneFix® intrauterine contraceptive system. Eur J Contracept Reprod Health Care 7: 24–30. [Crossref]













