Abstract
Methotrexate (MTX) is an essential chemotherapeutic medication that had diverse side effects on different organs. This study was performed to investigate the probable protective effect of Omega-3 fatty acids and β-carotene against MTX induced testicular toxicity in adult male rats. This experiment was done in ten weeks, five groups (10 animals for each) of adult male rats were used, control group (I) without treatment, MTX group (II) and the protected groups (III, IV and V) were injected with a single dose of MTX 20 mg/kg I.P in the start of the tenth week. Protected groups regime was as follow: - Omega-3 protected group (III) which received Omega-3 fatty acids at a dose 500 mg/kg orally for ten weeks. β-carotene protected group (IV) which received β-carotene at a dose 10 mg/kg orally for ten weeks. Combination of omega-3 fatty acids & β-carotene protected group (V) which received both omega-3fatty acids (of 500 mg/kg orally) and β-carotene (10 mg/kg orally) daily at the same time for ten weeks.
Blood samples were gathered for estimation of testosterone level. Testicular specimens were subjected to morphometric, histological and immunohistochemical examination. MTX-treated group (II) displayed marked reduction in number populace of spermatocytes, atrophy and germ cell degeneration. Appearance of multinucleated giant cells and significant increase in apoptotic cells was observed. MTX -induced histopathological changes were ameliorated in the protected groups with Omega-3 fatty acids and β-carotene. Our results indicate that Omega-3fatty acids and β-carotene may be beneficial in reducing the side effects associated with MTX administration.
Key words
methotrexate, Omega-3fatty acids, β-carotene, apoptosis, testis
Introduction
Numerous investigators were documented the generous toxicity of chemotherapeutic drugs on different organs of the body. MTX, is a folic acid antagonist, is utilized as cytotoxic agent as a part of the treatment of cancer and leukemia and different malignancies and in addition in the inflammatory diseases, for example, psoriasis and rheumatoid arthritis in low doses [1-3]. There was a plenty of proof that MTX instigates histopathological changes in the proliferative and germinative cells, such as testes which may lead to infertility [4]. Many studies have demonstrated that MTX toxicity was because of the generation of reactive oxygen species (ROS) [5,6]. Many antioxidants have been accounted for to improve the MTX-induced testicular histopathological damages. Omega-3 fatty acids were proven to have valuable effect against cardiovascular illnesses, lipid perioxidation, protein cross linking, DNA mutation and homeostasis were all due to its antioxidant activity [7]. β-carotene displays the antioxidant effect by suppressing singlet oxygen, scavenging peroxide radicals, therefore reacting with peroxy radicals, thus stabilizing membrane lipids from free radical attack [8,9]. This study was performed at the histopathological and Immunohistochemical level to investigate the protective effects of Omega-3 fatty acids and β-carotene against the MTX- related testicular alterations in male rats.
Materials and methods
Animals
Experiments were achieved on fifty adults (age 6-7 months) healthy Sprague-Dawley male albino rats of body weight 180 to 200g. which were obtained from Animal House of Zoology Department, Faculty of Sciences, Suez Canal University. They were housed under standard strict hygienic conditions; fed standard laboratory feed and had free access to water. Rats were maintained in a room temperature of 20-25◦C and exposed to12 hour light/dark cycle. Before the beginning of the experiment all animals were acclimatized for 1 week. Experimental protocol was approved in accordance with the Guidelines of Animal ethics council of the Faculty of Veterinary Medicine, Suez Canal University (Approval no.2016089).
Drugs
MTX was purchased from Ebewe Pharma Company (Egypt) and was dissolved in 0.9% saline. Omega-3 polyunsaturated fatty acids were purchased from Sedico Pharma Company (Egypt) and were obtained as gelatinous capsules. β- Carotene was purchased from Mepaco Pharma Company (Egypt) as gelatinous capsules. Both drugs were administrated by gavage.
Experimental protocol
Animals were divided equally into five groups (n=10 for each group) as follows: Control group (I) not subjected to any treatment, MTX – treated Group (П) and protective groups (III, IV and V), Rats were injected with a single dose of MTX (20 mg/kg i.p), at the start of the last tenth week of the experiment, according to the previous studies [6,10]. Omega -3fatty acids protective group (III), Rats were first given Omega-3fatty acids (orally via stomach gavage) at a dose 500 mg/kg, for ten weeks [11]. β-carotene protective group (IV), Rats were first given β- carotene orally via stomach gavage at a dose of 10 mg/kg daily for ten weeks. Combination of Omega-3 fatty acids & β-carotene group (V), at the same period, Rats were given both omega-3 (500 mg/kg orally) and β-carotene (10 mg/kg orally) daily for ten weeks.
Biochemical analysis
Estimation of serum testosterone was done at the end of tenth week of the experiment, blood samples were obtained from tail vein; each sample was collected in plain centrifuge tube and put on inclined position for 20 minute at room temperature. They were centrifuged at 3000 rpm for 10 minutes, then the clear serum of each sample was separated carefully, collected, and stored in Eppendorf tubes at 20oC until estimation of serum testosterone. Serum testosterone concentration was determined by using enzyme-linked immunoassay (ELISA) [12].
Tissue preparation
At the end of the tenth week of the experiment, rats were anesthetized with ether and killed by cervical dislocation. Testes were isolated and fixed in 10% neutral buffered formalin for histopathological examination and immunohistochemistry. Testicular sections were washed several times in dehydrated ascending series of ethyl alcohol, cleared in xylol and then embedded in three changes of pure paraffin wax. For histopathological examination, testicular sections were subjected to routine histological technique and stained with Harri's Hematoxylin and Eosin (H&E) according to carson (1997)[13].
Immunohistochemistry
For caspase-3 immunostaining, testicular paraffin embedded sections were deparaffinized, microwaved and incubated in 3% H2O2, then washed in PBS. Sections were incubated in normal goat serum at 37°C for blocking of non-specific antibody binding. They were subjected to biotinylated primary antibodies (Rabbit anti-caspase-3, diluted in 1:1000, Abcam, Ltd., USA). Then, they were incubated with biotinylated secondary antibody peroxidase-conjugated goat anti-rabbit IgG (1:1000). Streptavidin-biotin complex technique (ABC kit, Vector laboratories, USA) was used. Immunostaining reaction was labeled with diaminobenzidine (DAB) as a chromogen and counterstained with Mayer's Hematoxylin. Images were inspected with light microscope and captured using a digital camera (Olympus DB25- Japan). Assessment of immunostaining expression of caspase-3 in the testicular sections was performed using image J program.
Morphometric studies
Diameters of seminiferous tubules and distances of interstitial spaces were measured using Image-J program. Five slides stained with Hematoxylin and eosin (H&E) per animal were evaluated. The diameter of 25 seminiferous tubules was calculated in 5 fields (5 seminiferous tubules per field). In a similar manner the interstitial space measured between two consecutive seminiferous tubules with Mag. X 100.
Statistical analysis
One way analysis of variance (ANOVA) test was applied for the statistical analysis for the present data of all experimental groups. The result was considered to be significant when p<0.05 and highly significant when p<0.01.
Results
Evaluation of serum level of testosterone
Testosterone level was significantly reduced in treated group with MTX-treated group (II) (p<0.05), however, testosterone level was increasing significantly in omega 3 fatty acids protected group(III) rather than β carotene protected group (IV) (Figure 1).
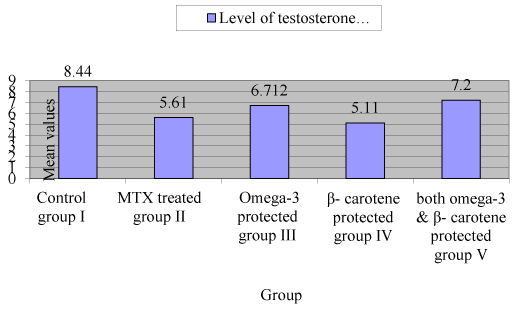
Figure 1. Level of testosterone in control and experimental groups (mean ± SEM)
Morphometric analysis
The diameter of seminiferous tubules was recorded to be significantly decreased (p<0.05) in with MTX treated group as compared to control and protective groups (III, IV and V) (Figure 2). Significant differences (p<0.05) were also observed increasing Interstitial spaces were measured between control and MTX treated group and other groups. Correlation was significant between different experimental groups and MTX group in changes in diameter of seminiferous tubules, interstitial space and level of testosterone (Table 1 and Figure 3-5).
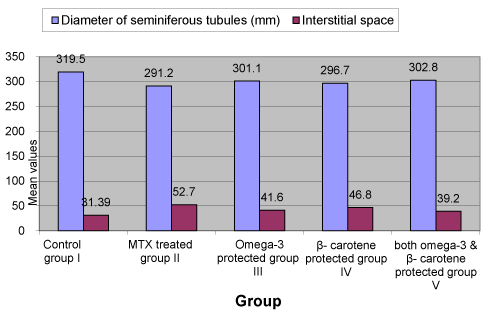
Figure 2. Diameter of seminiferous tubules and interstitial spaces in both control and experimental groups
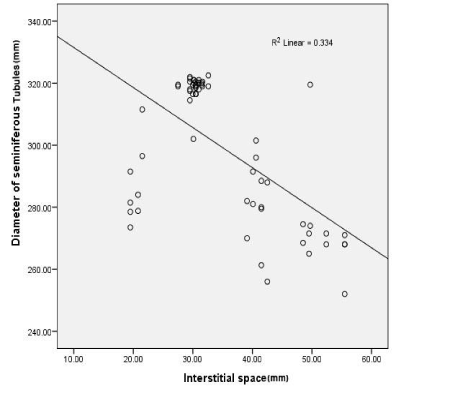
Figure 3. Shows a straight line representing the correlation between different groups changes in an interstitial space and diameter of seminiferous tubules
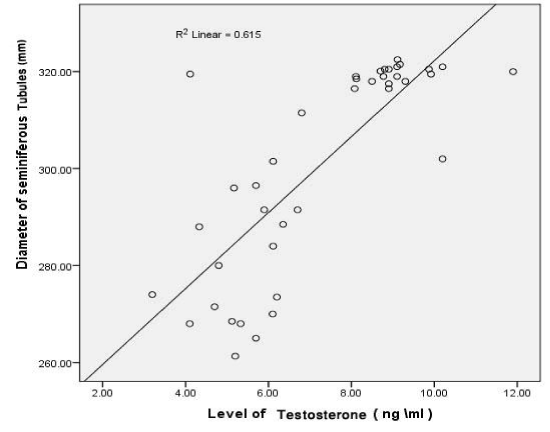
Figure 4. Shows a straight line representing the correlation between different groups changes in level of testosterone and diameter of seminiferous tubules
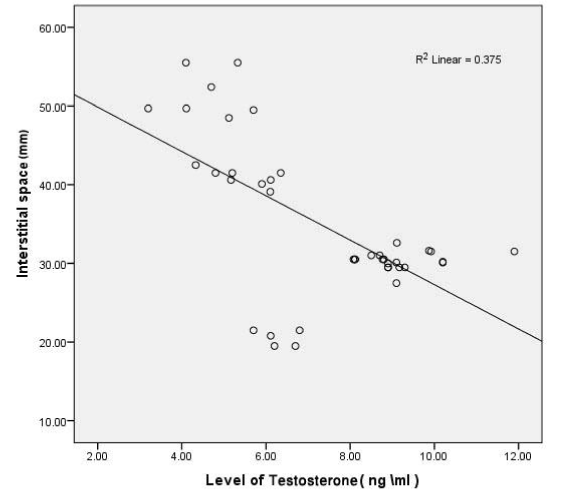
Figure 5. Shows a straight line representing the correlation between different groups changes in level of testosterone
Correlations |
|
Diameter of seminiferous tubules(mm) |
Interstitial spaces(mm) |
Level of Testosterone(ng/ml) |
Pearson Correlation |
0.612 |
- 0.578** |
0.784** |
Sig. (2-tailed) |
1 |
0.0001 |
0.0001 |
N |
64 |
64 |
40 |
Table 1. Correlation between different experimental groups and MTX group in changes in diameter of seminiferous tubules, interstitial space and level of testosterone. ** Correlation is significant at the 0.01 level (2-tailed). reveres relationship in correlation test
Histological results
Control group (I): Examination of H&E stained sections of control group revealed normal histological structure; seminiferous tubules were lined by sertoli cells and spermatogenic cell stages (Figure 6A1). Clusters of Leydig cells and blood capillaries were randomly scattered in the interstitial spaces (Figure 6A1 and 6A2).
MTX – treated group (II): significant decrease in the diameters of seminiferous tubules. Marked depletion in germ cell population, disorganization of germinal epithelium and degeneration of spermatogenic stages in the majority of the seminiferous tubules were demonstrated (Figure 6B1). Germ cell stages were devoid of secondary spermatocytes and spermatids (Figure 6B2). Apparent, multivesicular giant cells of different sizes were randomly distributed throughout the germinal epithelium which was associated with distinct large vacuoles (Figure 6B1 and 6B2). Seminiferous tubules showed detached basement membranes and peritubular tissue. Noticeable increase in the distances of interstitial spaces was observed which was occupied with congested blood vessels, hemorrhage, and exudates (Figure 6B1and 6B2).
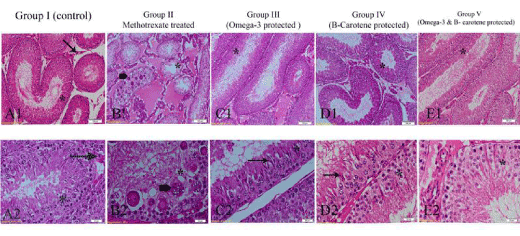
Figure 6. Photomicrographs of the testis of different experimental groups, stained with H&E. (A1&A2) Control group (I) showing normal histological structure of seminiferous tubules showing spermatogenic cell stages (*) and Leydig cells (arrow). (B1&B2) MTX-treated group (II) showing disorganization, depletion of spermatogenic cell stages (*) and appearance of multinucleated giant cells (thick arrow). noticeable eosinophilic exudates in interstitial spaces. (C1&C2) Omega-3 fatty acid protected group (III) showing reorganization of germinal cell stages (*) with elongated spermatids (arrow). (D1&D2) β-carotene protected group (IV) showing nearly normal structure (*) with elongated spermatids (arrow). (E1&E2) both Omega- 3 fatty acids and β- carotene group (V) showing germinal cell stages (*) with reduction of elongated spermatids. Fig. A1, B1, C1, D1 and E1 scale bar 100 µm and Fig. A2, B2, C2, D2 and E2 scale bar 20 µm.
Omega -3 fatty acids protected group (III)
Prominent dramatic organization of spermatogenic stages was evident; spermatogonia, primary & secondary spermatocytes, elongated spermatids and spermatozoa were observed. There were no signs of degeneration of germ cell layer (Figure 6C1 and 6C2). Sperm bundles were occupying luminae of seminiferous tubules. Germinal epithelium was devoid of multinucleated giant cells (Figure 6C1 and 6C2).
β -carotene protected group (IV)
Sections pretreated with β- carotene exhibited nearly normal structural architecture of the testis; all the organized spermatogenic stages were observed with apparent elongated spermatids (Figure 6D2). Germinal epithelium was devoid of multinucleated giant cells (Figure 6 D1 and 6D2). The luminae of seminiferous tubules were occupied with sperm bundles. Interstitial space showed congestion of blood vessels and exudates similar to group II (methotrexate- treated group) (Figure 6 D1and 6D2).
Combination of omega- 3 fatty acids and β- carotene group (V)
Testicular sections pretreated with both omega-3fatty acids and β-carotene showed systematized spermatogenic stages except that elongated spermatids were reduced (Figure 6 E1and 6E2). No significant differences between the protected group with both Omega- 3fatty acids and β-carotene (group V) and the protected group with either omega- 3 fatty acids (group III) or β-carotene (group IV) alone except that the reduction of elongated spermatids in group V.
Immunohistochemical result of Caspase-3 apoptotic factor
In control group (I), immunostaining reaction of spermatogenic cells and Leydig cells was negative (Figure 7a). Strong staining reaction was apparent in methotrexate-treated group (II); it was more prominent in the spermatogenic cells, sertoli cells, multinucleated giant cells and Leydig cells (Figure 7b). Faint Immunostaining reaction of spermatogenic cells and moderate reaction of Leydig cells were observed in pretreated groups with Omega-3 fatty acids group III (Figure 7c), β-carotene group IV (Figure 7d), and both Omega-3 fatty acids & β-carotene group V (Figure 7e).
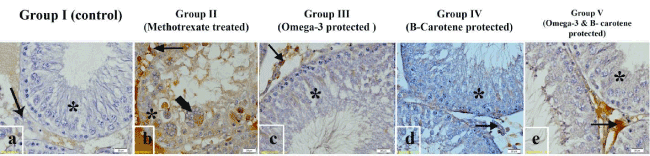
Figure 7. Photomicrographs of the testis of different experimental groups incubated with caspase-3 monoclonal antibody. (a) Control group (I) showing negative immunostaining affinity of spermatogenic stages (*) and Leydig cells (arrow). (b) MTX-treated group (II) showing strong immunostaining of spermatogenic cells (*), multinucleated giant cells (thick arrow) and Leydig cells (arrow) different experimental groups. (c) Omega-3 fatty acid protected group (III), (d) β-carotene protected group (IV) and (e) both Omega- 3 fatty acids and β- carotene group (V) showing faint Immunostaining reaction in the spermatogenic cells (*) Leydig cells were less intense in the(d) β-carotene protected group (IV). Scale bar 20 µm.
In studying the number of apoptotic germ cells immunostained with caspase 3, there was a significant difference between the groups as demonstrated by the ANOVA P value <0.05. There was a significant difference between control group (I) and MTX group (II) and a significant difference between MTX group (II) and rest of groups. There was no significant difference between group III, group (IV) and group (V) P value > 0.05 (Figure 8).
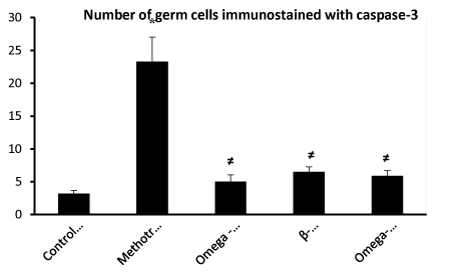
Figure 8. * Significantly different from control group (I) P< 0.05.
Discussion
MTX is a folic acid antagonist agent; it belongs to the antimetabolites group of cytotoxic and immunosuppressant drugs [14,15]. Many reports have been published on testicular damage caused by chemotherapeutic drugs [3,16]. They could lead to Permanent azoospermia and infertility [17].
This study demonstrated that testosterone level decreased in MTX-treated group (II) in comparison to control group (I) and protected groups with Omega-3fatty acids group (III), β-carotene group (IV) and a combination of them group (V). Some authors revealed the capacity of β- carotene to restore the testosterone level after the apparent decrease induced by titanium dioxide nanoparticles [18]. Administration of Omega-3 fatty acids for the reestablishing of serum testosterone level reduction induced by MTX was documented [19].
In the present study, diameter of seminiferous tubules was diminished in MTX treated group compared to control and protective groups. This could be recognized with the medication effect of MTX [20,21]. Furthermore, MTX toxicity could influence cell parts, seminiferous tubules diameter and interstitial space throughout spermatogenesis [22].
Our results showed that MTX administration causes testicular damages which demonstrated by disorganization of germinal epithelium, depletion in germ cell stages, and decrease in sperm population and also increase in apoptotic degenerated cells. This was in concurrence with [3,23] who stated that in MTX treated group, there were widespread loss of spermatozoa, disorganization of spermatogenic stages and extensive apoptotic germinal cells. MTX induced histopathological changes could be clarified by [24] who expressed that MTX and its dynamic metabolite block tetrahydrofolate synthesis through binding to the folic acid site on the catalyst dihydrofolate reductase. This activity results in the decrease of nucleotide precursors, and the suppression of DNA, RNA, protein synthesis and cellular proliferation.
Others researchers were attributed to Sertoli cell damage induced by MTX since the nutritional and structural support of germ cells is supported by sertoli cells [25]. Some authors proposed that sloughing of germinal epithelium may be brought about by downregulation of expression of cell adhesion (e.g. cadherin) in Sertoli cells [26]. It might be because of the diverse effect of chemicals on the cytoskeleton of Sertoli cells [27].
Moreover, MTX-treated group (II) displayed multinucleated giant cells in the germinal epithelium that had a strong immunostaining affinity to caspase-3. It is conceivable that multinucleated giant cells are a cluster of degenerated germ cells [21,28].
Besides, Oxidative stress could explain histopathogenesis of MTX-induced testicular changes and marked immunostaining intensity of apoptotic cells [29]. Oxidative stress causes diminishing in mitochondria capacities, cytochrome c release, and consequently caspase activation [30]. MTX stimulates oxidative stress by lessening the activities and the effectiveness of the antioxidant enzyme protection system [31] which reduced testicular functions and disruption of sperm development [30,32]. In the protected groups with omega 3 fatty acids, β- carotene and combination of them as an antioxidant, a significant improvement in the histopathological features of the testis was observed. Remarkable testicular histopathological damages were ameliorated that manifested through rearrangement of germinal epithelium and an increase of sperm populace. Germinal epithelium was devoid of multinucleated giant cells and degenerated cells. In addition to, noticeable reduction in the immunostaining intensity (caspase-3) of germinal epithelial cells was evident. This was in agreement with [30] and [23] who reported that β-carotene decreased testicular injury and germ cell apoptosis in the testis after testicular ischemia/reperfusion damage. The protective activity of Omega-3 fatty acids against MTX- induced testicular damage could be attributed to its antioxidant properties
2021 Copyright OAT. All rights reserv
b-carotene has been accounted for attenuate tissue oxidant catalysts in various animal models [23,33-35]. b-carotene is a precursor of vitamin A which is act as potent antioxidant agent that block the free radicle formation or scavenger them [21]. The current study confirmed that pretreatment with β-carotene clearly diminishes the degenerative apoptotic alterations in germinal epithelium. [36] recommended that protection of β-carotene could owe to its antiapoptotic activity. [32] reported that nuclear structural changes as fragmentation and denaturation of DNA, and resulting apoptosis were brought on by oxidative stress. [37] revealed that β-carotene diminishes restraint of purine and pyrimidine synthesis of DNA. The protective effect of vitamin A against MTX on the small intestine might be clarified by stimulus of DNA and RNA biosynthesis [31]. In conclusion, we have presented noteworthy evidence that oral supplementation with Omega3fatty acids, β-carotene or combination of them as antioxidants could reestablish testicular histopathological alterations associated with administration of Methotrexate as chemotherapy.
References
- Babiak RM, Campello AP, Carnieri EG, Oliveira MB (1998) Methotrexate: pentose cycle and oxidative stress. Cell Biochem Funct 16: 283-293. [Crossref]
- Bayram M, Ozogul C, Dursun A, Ercan ZS, Isik I, et al. (2005) Light and electron microscope examination of the effects of methotrexate on the endosalpinx. Eur J Obstet Gynecol Reprod Biol 120: 96-103. [Crossref]
- Sönmez MF, Çilenk KT, Karabulut D, Ünalmış S, Deligönül E, et al. (2016) Protective effects of propolis on methotrexate-induced testis injury in rat. Biomed Pharmacother 79: 44-51. [Crossref]
- Daggulli M, Dede O, Utangac MM, Bodakci MN, Hatipoglu NK, et al. (2014) Protective effects of carvacrol against methotrexate-induced testicular toxicity in rats. Int J Clin Exp Med 7: 5511-5516. [Crossref]
- Sener G, Ekşioğlu-Demiralp E, Cetiner M, Ercan F, Sirvanci S, et al. (2006). L-Carnitine ameliorates methotrexate-induced oxidative organ injury and inhibits leukocyte death. Cell Biol Toxicol 22: 47-60. [Crossref]
- Uraz S, Tahan V, Aygun C, Eren F, Unluguzel G, et al. (2008) Role of ursodeoxycholic acid in prevention of methotrexate-induced liver toxicity. Dig Dis Sci 53: 1071-1077. [Crossref]
- Rodriguez-Leyva D, Dupasquier CM, McCullough R, Pierce GN (2010) The cardiovascular effects of flaxseed and its omega-3 fatty acid, alpha-linolenic acid. Can J Cardiol 26: 489-496. [Crossref]
- Vardi N, Parlakpinar H, Cetin A, Erdogan A, Cetin Ozturk I (2010) Protective effect of beta-carotene on methotrexate-induced oxidative liver damage. Toxicol Pathol 38: 592-597. [Crossref]
- Fujii J, Iuchi Y, Matsuki S, Ishii T (2003) Cooperative function of antioxidant and redox systems against oxidative stress in male reproductive tissues. Asian J Androl 5: 231-242. [Crossref]
- Bathon JM, Martin RW, Fleischmann RM, Tesser JR, Schiff MH, et al. (2000) A comparison of etanercept and methotrexate in patients with early rheumatoid arthritis. N Engl J Med 343: 1586-1593. [Crossref]
- Brenna JT, Salem N Jr, Sinclair AJ, Cunnane SC; International Society for the Study of Fatty Acids and Lipids, ISSFAL (2009) alpha-Linolenic acid supplementation and conversion to n-3 long-chain polyunsaturated fatty acids in humans. Prostaglandins Leukot. Essent. Fatty Acids 80: 85-91. [Crossref]
- Singer F, Zumoff B (1992) Subnormal serum testosterone levels in male internal medicine residents. Steroids 57: 86-89. [Crossref]
- Carson F (1997) Hfistotechnology a self-intructuctional text (ASCP).
- Mueller-Koch Y, Vogelsang H, Kopp R, Lohse P, Keller G, et al. (2005) Hereditary non-polyposis colorectal cancer: clinical and molecular evidence for a new entity of hereditary colorectal cancer. Gut 54: 1733-1740. [Crossref]
- Sukhotnik I, Mogilner JG, Karry R, Shamian B, Lurie M, et al. (2009) Effect of oral glutamine on enterocyte turnover during methotrexate-induced mucositis in rats. Digestion 79: 5-13. [Crossref]
- Ben Arush MW, Solt I, Lightman A, Linn S, Kuten A (2000) Male gonadal function in survivors of childhood Hodgkin and non-Hodgkin lymphoma. Pediatr Hematol Oncol 17: 239-245. [Crossref]
- Kim JC, Kim KH, Chung MK (1999) Testicular cytotoxicity of DA-125, a new anthracycline anticancer agent, in rats. Reprod. Toxicol 13: 391-397. [Crossref]
- Orazizadeh M, Daneshi E, Hashemitmar M, Absalan F, Khorsandi L (2015) Protective effect of beta-carotene against titanium dioxide nanoparticles induced apoptosis in mouse testicular tissue. Andrologia 47: 816-825. [Crossref]
- Bansal AK, Bilaspuri GS (2010) Impacts of oxidative stress and antioxidants on semen functions. Vet Med Int 686137. [Crossref]
- Gulgun M, Erdem O, Oztas E, Kesik V, Balamtekin N (2010) Proanthocyanidin prevents methotrexate-induced intestinal damage and oxidative stress. Exp Toxicol Pathol 62: 109-115. [Crossref]
- Yüncü M, Bükücü N, Bayat N, Sencar L, Tarakçioğlu M (2015) The effect of vitamin E and L-carnitine against methotrexate-induced injury in rat testis. Turk J Med Sci 45: 517-525. [Crossref]
- Armagan A, Uzar E, Uz E, Yilmaz HR, Kutluhan S, et al. (2008) Caffeic acid phenethyl ester modulates methotrexate-induced oxidative stress in testes of rat. Hum Exp Toxicol 27: 547-552. [Crossref]
- Vardi N, Parlakpinar H, Ates B, Cetin A, Otlu A (2009) Antiapoptotic and antioxidant effects of beta-carotene against methotrexate-induced testicular injury. Fertil Steril 92: 2028-2033. [Crossref]
- Koppelmann T, Pollak Y, Mogilner J, Bejar J, Coran AG, et al. (2013) Reversal of severe methotrexate-induced intestinal damage using enteral n-3 fatty acids. Br J Nutr 109: 89-98. [Crossref]
- Lim J, Miller MG (1997) The role of the benomyl metabolite carbendazim in benomyl-induced testicular toxicity. Toxicol Appl Pharmacol 142: 401-410. [Crossref]
- Kim JM, Ghosh SR, Weil AC, Zirkin BR (2001) Caspase-3 and caspase-activated deoxyribonuclease are associated with testicular germ cell apoptosis resulting from reduced intratesticular testosterone. Endocrinology 142: 3809-3816. [Crossref]
- Sayim F (2007) Histopathological effects of dimethoate on testes of rats. Bull Environ Contam Toxicol 78: 479-484. [Crossref]
- Yin Y, DeWolf WC, Morgentaler A (1998) Experimental cryptorchidism induces testicular germ cell apoptosis by p53-dependent and -independent pathways in mice. Biol Reprod 58: 492-496.
- Revel A, Raanani H, Younglai E, Xu J, Han R, et al. (2001) Resveratrol, a natural aryl hydrocarbon receptor antagonist, protects sperm from DNA damage and apoptosis caused by benzo(a)pyrene. Reprod Toxicol 15: 479-486. [Crossref]
- Uguralp S, Usta U, Mizrak B (2005) Resveratrol may reduce apoptosis of rat testicular germ cells after experimental testicular torsion. Eur J Pediatr Surg 15: 333-336. [Crossref]
- Elbakary NA, Soliman GM, Tawfik SM, Zaher SM (2014) Evaluation of the possible protective role of vitamin A on methotrexate-induced changes on the jejunal mucosa of adult male albino rat: Histological and immunohistochemical study. Journal of Microscopy and Ultrastructure 2: 77-93.
- Prahalathan C, Selvakumar E, Varalakshmi P (2005) Protective effect of lipoic acid on adriamycin-induced testicular toxicity. Clin Chim Acta 360: 160-166. [Crossref]
- Kheir-Eldin AA, Motawi TK, Gad MZ, Abd-ElGawad HM (2001) Protective effect of vitamin E, beta-carotene and N-acetylcysteine from the brain oxidative stress induced in rats by lipopolysaccharide. Int J Biochem Cell Biol 33: 475-482. [Crossref]
- El-Demerdash FM, Yousef MI, Kedwany FS, Baghdadi HH (2004) Cadmium-induced changes in lipid peroxidation, blood hematology, biochemical parameters and semen quality of male rats: protective role of vitamin E and beta-carotene. Food Chem Toxicol 42: 1563-1571. [Crossref]
- Vardi N, Parlakpinar H, Ozturk F, Ates B, Gul M, et al. (2008) Potent protective effect of apricot and beta-carotene on methotrexate-induced intestinal oxidative damage in rats. Food Chem Toxicol 46: 3015-3022. [Crossref]
- Stahl W, Sies H (2003) Antioxidant activity of carotenoids. Mol Aspects Med 24: 345-351. [Crossref]
- Tsurusawa M, Saeki K, Fujimoto T (1997) Differential induction of apoptosis on human lymphoblastic leukemia Nalm-6 and Molt-4 cells by various antitumor drugs. Int J Hematol 66: 79-88. [Crossref]








