Abstract
We detected a first case of 6-Pyruvoyl-tetrahydropterin synthase deficiency in Neuropediatric department mongi Ben Hmida of Tunisia. Genetic analyses of PTS gene demonstrated a homozygous mutation; treatment had been started at the age of 3years.
Keywords
Biopterin, Hyperphenylalaninemia, Neopterin, Tetrahydrobiopterin, Pyruvoyl-tetrahydropterin synthase deficiency
Introduction
Tetrahydrobiopterin BH4 acts as a cofactor for phenylalanine hydroxylase as well as for tyrosine hydroxylase and tryptophan hydroxylase, which are required for the synthesis of dopamine and serotonin, respectively [1]. BH4 synthesis from GTP in humans requires some enzymes including dihydropterin redustase (DHPR) and 6-Pyruvoyl-tetrahydropterin synthase (PTS; EC 4.6.1.10; MIM# 261640) [2]. PTS deficiency is a major cause of BH4-deficient hyperphenylalaninemia (HPAP) [3]. It also causes neurotransmitters synthesis decline [4]. The biological profile is characterized by high blood phenylalanine concentration, low urinary total biopterin and high neopterin:biopterin ratio, as well as decreased neurotransmitter metabolites homovanillic acid and 5-hydroxyindoleacetic in the cerebrospinal fluid [5]. Diagnosis is confirmed by measuring PTS activity in erythrocytes and identification of PTS gene mutation [6-8]. In this study, we report the first Tunisian case with PTS deficiency.
Case Report
A one years old boy presented to Neuropediatric department with psychomotor delay. He is the second child of first degree consanguineous healthy parents. His sister died at the age of 18 months from a similar illness. He had a personal history of perinatal asphyxia, recurrent respiratory infections and hyperthermia. Examination showed irritability, axial hypotonia, spastic tetraparesis, dystonia, oculogyre crisis and increased sweating. Brain MRI showed frontal cortical atrophy and periventricular T2 hyperintensity (Figure 1).
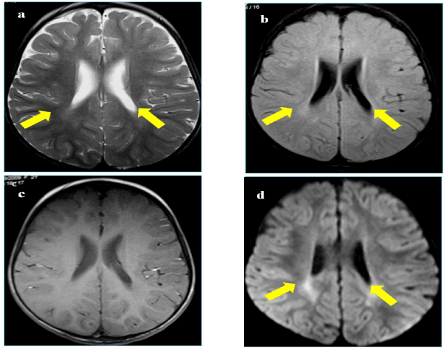
Figure 1. a. Cerebrospinal MRI showing an hypersignal T2; b. FLAIR; c. prominent periventricular white matter posteriorly and appearing in isosignal T1; d. with restriction of ADC on the weighted diffusion sequence as b1000
Laboratory findings on admission were as follows: Guthrie test showed elevated blood phenylalanine, 31 mg/dL (normal values, < 3). Urinary pterin concentrations measured by high performance liquid chromatography with low biopterin, 194 nmol/mmol creatinine (normal values, 3500-5000), high neopterin, 24556 nmol/mmol creatinine (normal values, 3000-5000) and elevated neopterin:biopterin ratio, 127 (normal values, ≤ 1.22). Blood activity of DHPR was normal, 1.6 nmol/min/mg hemoglobin (normal values, 1-5). Genetic analysis of PTS gene demonstrated a homozygous mutation c.169_171del GTG (Val57del) confirming the diagnosis of PTS deficiency.
The Patient underwent BH4 treatment (5mg/kg/j) at the age of 3 years resulting in a mild clinical improvement. Blood phenylalanine was reduced under treatment to less than 1 mg/dL at age of 4 years. No abnormalities in height or weight were observed in our patients.
The three-nucleotide deletion identified in this patient was assigned as 169-171del GTG. Such deletion leads to an in-frame deletion of valine at codon 57 (V57del) and had been identified in Caucasian and Chinese patient
[9-11].
2021 Copyright OAT. All rights reserv
In absence of neonatal screening in Tunisia, diagnosis of PTS deficiency should be considered in children with phenylketonuric phenotype associated to neurological signs and symptoms related to impaired catecholamines and serotonin synthesis (movement disorders, microcephaly, seizures, dysautonomic signs). Early diagnosis allows initiation of appropriate therapy and genetic counseling.
Declaration of interest
The authors declare no conflict of interest.
References
- Opladen T, Abu Seda B, Rassi A, Thöny B, Hoffmann GF, et al. (2011) Diagnosis of tetrahydrobiopterin deficiency using filter paper blood spots: further development of the method and 5 years’ experience. J Inherit Metab Dis 34: 819-26. [Crossref]
- Shintaku H (2002) Disorders of tetrahydrobiopterin metabolism and their treatment. Curr Drug Metab 3: 123-31. [Crossref]
- Blau N, Barnes I, Dhondt JL (1996) International database of tetrahydrobiopterin deficiencies. J Inher Metab Dis 19: 8-14. [Crossref]
- Scriver CR, Kaufman S, Eisensmith RC, Woo SLC (1995) The hyperphenylalaninemias. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill p: 1015-1075.
- Longo N (2009) Disorders of biopterin metabolism. J Inherit Metab Dis 32: 333-342. [Crossref]
- Thony B, Leimbacher W, Blau N, Harvie A, Heizmann CW (1994) Hyperphenylalaninemia due to defects in tetrahydrobiopterin metabolism-molecular characterization of mutations in 6-pyruvoyl-tetrahydropterin synthase. Am J Hum Genet 54: 782-792. [Crossref]
- Lee HH, Mak CM, Poon GW, Wong KY, Lam CW (2014) Cost-benefit analysis of hyperphenylalaninemia due to 6-pyruvoyl-tetrahydropterin synthase (PTPS) deficiency: for consideration of expanded newborn screening in Hong Kong. J Med Screen 21: 61-70. [Crossref]
- Ye J, Yang Y, Yu W, Zou H, Jiang J, et al. (2013) Demographics, diagnosis and treatment of 256 patients with tetrahydrobiopterin deficiency in mainland China: results of a retrospective, multicentre study. J Inherit Metab Dis 36: 893-901. [Crossref]
- Oppliger T, Thony B, Nar H, Burgisser D, Huber R, et al. (1995) Structural and function consequences of mutation in 6 pyruvoyltetrahydropterin synthase causing hyperphenylalaninemia in humans-phosphorylation is a requirement for in vivo activity. J Biol Chem 270: 29498-29506. [Crossref]
- Oppliger T, Thony B, Kluge C, Matasovic A, Heizmann CW, et al. (1997) Identification of mutations causing 6-pyruvoyl-tetrahydropterin synthase deficiency in four Italian families. Hum Mutat 10: 25-35. [Crossref]
- Liu TT, Chang YH, Chiang SH, Yang YL, Yu WM, et al. (2001) Identification of three novel 6-pyruvoyl-tetrahydropterinsynthase gene mutations (226C>T, IVS3+1G>A, 116-119delTGTT) in Chinese hyperphenylalaninemia caused by tetrahydrobiopterin synthesis deficiency. Hum Mutat 18: 83.

