Abstract
Germ cell tumors primarily affect adolescents and young adult men. They occur much less commonly in older patients. The definition of elder age in this kind of tumors, as the detailed clinical and treatment characteristics in this age group are lacking. According to the literature, germ cell tumors are infrequent in men aged more than 50 years old, and there is a predominance of seminomatous histologic subtype. Here we report the case of a 56 years old male patient who was initially diagnosed with pure testicular seminoma at the age of 55. He underwent a right orchiectomy and didn’t receive an adjuvant treatment for a pure seminomatous germ cell tumor, staged pT1 with rete testis invasion. Unfortunately, he suffered from a retroperitoneal relapse of his disease 9 months after the surgery. He received a first line chemotherapy consisting of 4 cycles of Etoposide and Cisplatin. He tolerated very well the treatment with an excellent response and without developing any related toxicity. He is currently in complete remission on regular follow up. This case report and review of the literature are to emphasize on germ cell tumors diagnosed after the age of 50 years old. This category of patients should be treated with full curative surgical and cytotoxic treatments, as it is done in the younger population, in order to achieve a good overall response rate. However, they require careful observation and increased supportive care for better treatment tolerability and thus better outcome.
Key words
seminoma, germ cell tumor, elder
Introduction
Germ cell tumors (GCTs) are considered the most common solid tumors in young adults, but they are infrequent in the elder population [1]. The World Health Organization Health Organization (WHO) classification of tumors categorizes GCTs in adolescents and adults into 3 histologic groups: classic seminoma with pure histology, non-seminomatous GCT (NSGCT), comprising embryonal carcinoma, yolk sac tumor, choriocarcinoma, teratoma, mixed histologies (which can include seminoma) and spermatocytic seminoma [2]. Rates of seminoma slightly exceed those of NSGCT in most of the reported epidemiological surveys [3,4]. The incidence of seminoma and NSGCT differs across age groups: it is the highest between ages of 35 and 39 years for seminoma compared with 25 to 29 years for NSGCT. However, GCT are rare in older men with fewer than 10% and 2% of cases diagnosed at age 50 and 65, respectively [4].
Case report
A 56 years old gentleman, known to have a stage II pulmonary sarcoidosis since 4 years, was diagnosed, in another institution, with a right testicular pure seminoma at the age of 55 years old.
The patient felt a lump in his right testicle that was confirmed to be a mass on the testicular ultrasound. Lactate dehydrogenase (LDH), alfa-feto protein (AFP) and beta HCG (βHCG) were normal. The total body scan revealed the presence of known bilateral lung nodules with hilar and mediastinal lymphadenopathies secondary to his sarcoidosis. These latter were biopsied and the non-caseating granulomas of sarcoidosis were confirmed.
He underwent a right radical inguinal orchiectomy. The pathology report came in favor of a pure seminomatous germ cell tumor, staged pT1, with rete testis invasion and without any peri-vascular involvement.
The patient didn't receive any adjuvant treatment and was kept on close follow up and active surveillance.
Unfortunately, on the third follow up evaluation and 9 months after the diagnosis, the chest abdomen and pelvis CT scan showed an interval appearance of multiple retroperitoneal supra-centimetric lymphadenopathies: a 2.5×2 cm lymph node (LN) in the liver hilum, a 3.4 x 2 cm magma of LN in the inter aortocaval space and a 1.2 cm LN anteriorly to the inferior vena cava (Figure 1A). However, the bilateral lung nodules, the parenchymal infiltrates associated with the known mediastinal lymphadenopathies secondary to sarcoidosis were stable.
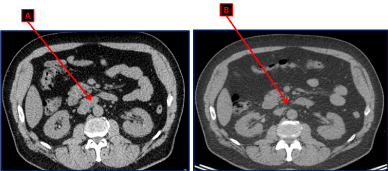
Figure 1. CT scan of the abdomen and pelvis showing the magma of retroperitoneal lymphadenopathies in the aortocaval space (A) upon recurrence. These lymph nodes disappeared after 4 cycles of chemotherapy with Cisplatin and Etoposide (B).
He came to our institution after this suspicion of recurrence of his disease. An abdomen and pelvis MRI were done and confirmed the retroperitoneal disease. The FDG PET CT scan showed hypermetabolic retroperitoneal lymph nodes with non-metabolic mediastinal LN (Figure 2A).
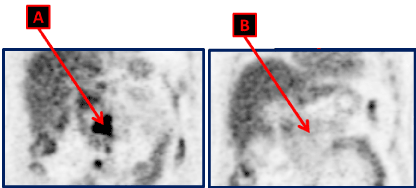
Figure 2. FDG PET CT scan done at the moment of the disease recurrence, showing hypermetabolic lymphadenopathies in the retroperitoneal space (A). They disappeared with complete metabolic response (B) on the FDG PET CT scan repeated after 4 cycles of Cisplatin and Etoposide.
The LDH was 390 U/L (normal value < 225 U/L). The rest of tumor markers consisting of AFP and βHCG were normal.
A laparoscopic biopsy of the inter aortocaval magma of lymph nodes was done. The pathology revealed a proliferation of cells with abundant cytoplasm, round shape nucleus and prominent nucleoli. Immunohistochemical analysis revealed a high positivity for OCT4 and CD30. Thus, the patient was diagnosed with a relapse of his known pure seminoma.
Consequently, he received a systemic chemotherapeutic treatment for his recurrent disease consisting of EP protocol. He had good cardiac and renal functions. View the history of sarcoidosis, it was decided to treat him with intravenous Etoposide 100 mg/m2 over 5 days and Cisplatin 20 mg/m2 over 5 days, without Bleomycin. Granulocyte colony stimulating factor (GCSF) support was given after each cycle. The LDH normalized after the first cure.
Four cycles of chemotherapy were administered with good clinical and biological tolerance. He didn’t develop any toxicity except for grade I nausea without vomiting, lasting few days after the end of the treatment.
After the fourth cycle, a chest abdomen and pelvic CT scan, an abdominal MRI and a PET CT scan were repeated. The known bilateral lung nodules and mediastinal LN secondary to his well-known sarcoidosis were stable. However, all the retroperitoneal lymphadenopathies that were seen before starting the chemotherapy, have disappeared (Figures 1B and 3). Furthermore, the PET CT scan confirmed the disappearance of the retroperitoneal disease, and revealed a complete metabolic response (Figure 2).
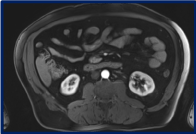
Figure 3. Abdomen MRI done at the end of the chemotherapy confirming the disappearance of retroperitoneal lymph nodes.
Therefore, our patient had a diagnosis of right testicular pure seminomatous germ cell tumor at the age of 55 and suffered from a retroperitoneal recurrence 9 months later, at 56 years old. He received a standard cytotoxic chemotherapeutic regimen with 4 cycles of Etoposide and Cisplatin at full doses. The treatment was well tolerated and the patient is currently in complete remission on regular follow up: clinical, laboratory and radiologic evaluations every 3 months.
Discussion
Germ cell tumor is the most common solid tumor seen in young men aged between 15 and 34 years old. It is unusual in elder population [1]. 9000 cases of GCT are approximately diagnosed in the united states annually. More than 90% occur in patients under the age of 50 years. Distribution of histologic subtypes differs significantly with age. Seminoma constitute the most commonly diagnosed histologic subtype above the age of 50 years (64%), whereas NSGCT is the dominant subtype for men less than 50 years (63.2%). The ratio of NSGCT to seminoma predominance gradually decreases with advanced age. The switch to seminoma predominance was seen approximately at the age of 35 in most reviews of the literature. Thus, an age cutoff of 35 to 40 years may better distinguish between older and younger groups [5]. Moreover, the distribution of primary tumor sites at diagnosis also varies with age. Retroperitoneal primary GCT is highly more frequent among older men. Primary mediastinal disease is equal in both age groups. However, primary central nervous system localization is typically seen below the age of 35 years old [6-8].
Data on the clinical characteristics and outcome in the elder population are lacking in the literature. Prior
epidemiologic reviews on GCTs have primarily insisted on population-based studies without detailed clinical information. Besides, other prior studies have reported a certain correlation between older age and more advanced disease at diagnosis with inferior outcome to treatment [9]. Similarly, a histopathologic study of 50 men with GCTs diagnosed at the age of 60 years found larger primary
tumor size and more frequent lymphovascular invasion and rete testis involvement compared with historical controls of all ages. However, this study’s results were rejected in another recent retrospective study finding that 77% of the 60 patients diagnosed at 60 years old had a stage I disease upon presentation, comparable with the rates seen in younger patients [10-12].
D. Feldman and his colleagues underwent a retrospective analysis of their large single-institution database in order to address GCTs in elder population. 4235 patients diagnosed with GCT over a 20-year period at Memorial Sloan-Kettering Cancer Center were reviewed. The results confirmed all the previously known epidemiologic data, but their study didn’t allow a direct comparison of stage distribution for older versus younger men [13].
Concerning the treatment of GCT in elder population, data on systemic chemotherapy for generalized disease is very limited. The cure rate of GCTs is usually comprised between 85 and 90%. This success in the treatment is the result of multimodal therapy including surgery, radiotherapy and especially Cisplatin based chemotherapy. Platinum is highly effective in combination with other drugs, but it is well known to be associated with emesis, neurotoxicity and renal toxicity. For good-risk patients, four cycles of Etoposide and Cisplatin (EP protocol) or three cycles of Bleomycin, Etoposide, and Cisplatin (BEP protocol) are the regimens of choice with a durable response rate of more than 90%. Carboplatin does not cause Cisplatin's related toxicity despite being more myelotoxic [14,15]. In elder patients presenting with metastatic disease, the usage of Etoposide and Carboplatin was evaluated in many trials. The medical research council (MRC) demonstrated that Carboplatin was associated with 10% inferior progression free survival (71 v/s 81%) and a non-significant survival difference favoring the Cisplatin combination (84% vs 89%) [16]. In another multi-institutional study, Bajorin et al. showed equivalent response rates and survival benefit but inferior event free survival for the Carboplatin group [17]. German series and those of Royal Marsden Hospital evaluated single agent Carboplatin in metastatic seminoma: the disease-free survival rates were more than 70% associated with more than 90 % of survival rates. Thus, Carboplatin may be an option for patients who are not eligible for Cisplatin due to renal failure or other contraindications.
Advanced age patients have a significantly affected tolerability of first-line platinum-based chemotherapy. Men above 50 years suffer from frequent serious complications exceeding historical rates from phase 3 studies that were mainly conducted in the younger population [12,13,18]. Wheater and colleagues reported that among 15 patients diagnosed with GCT and treated with EP or BEP at 60 years old, 30% were not able to complete the planned chemotherapy despite prophylactic dose attenuations [10].
However, elderly patients derive a similar benefit from chemotherapy as younger subjects. The major concern is the functional disability and the associated comorbidities that increase with age and adversely affect the outcome. This topic was well developed in previous studies that focused on the geriatric population in other malignancies [19,20]. Even among patients without significant comorbidities, age-related decline in organ function can impact chemotherapy pharmacokinetics, increasing drug exposure and toxicity. Among age related changes, the hematopoietic reserve, which is well known to decrease with age, is a primary determinant of myelosuppression with cytotoxic therapy.
In the literature, febrile neutropenia occurred in 44% of patients aged more than 50 years old, treated with first-line platinum-based chemotherapy [21-23]. However, Wheater and colleagues found a slightly lower incidence of febrile neutropenia among patients with GCT who were treated at 60 years old, possibly due to their use of prophylactic dose attenuations [10].
There is also a well-known correlation between older age and the risk of Bleomycin pulmonary
toxicity, as it was demonstrated in a series of 800 GCT patients treated with Bleomycin-containing regimens. Pulmonary toxicity was more reported in those aged above 40 years old on the multivariate analysis [24]. These findings can be explained by the age related changes in pulmonary physiology associated with variation in Bleomycin pharmacokinetics, knowing that it is primarily renally excreted.
Conclusion
In conclusion, GCTs occur less frequently in elder population with classic seminoma germ cell tumor predominance above the age of 50 years old. Patients should be evaluated and treated in a similar way to the adult population, keeping in mind that the treatment related complications and toxicities increase with age. Nevertheless, prognosis remains excellent when treatment is adequately given. The outcome by itself is not affected by age. Thus, risk directed chemotherapy should be administered in full curative doses, when possible, while escalating supportive measurements, including prophylactic growth factor support in order to overcome the potential for greater toxicity.
References
- Foell K, Martens M, Izawa JI (2007) A rare case of classic testicular seminoma in an 86-year-old shows similar proliferation rate as in younger men. Urology 70: e7-e9. [Crossref]
- Carrière P, Baade P, Fritschi L (2007) Population based incidence and age distribution of spermatocytic seminoma. J Urol 178: 125-128. [Crossref]
- Chia VM, Quraishi SM, Devesa SS, Purdue MP, Cook MB, et al. (2010) International trends in the incidence of testicular cancer, 1973-2002. Cancer Epidemiol Biomarkers Prev 19: 1151-1159. [Crossref]
- Townsend JS, Richar2021 Copyright OAT. All rights reservf testicular cancer in the United States, 1999-2004. Am J Mens Health 4: 353-360. [Crossref]
- National Cancer Institute. Cancer of the testis (invasive). Age-adjusted SEER Incidence Rates by Year, Race and Age.
- Berney DM, Warren AY, Verma M, Kudahetti S, Robson JM, et al. (2008) Malignant germ cell tumours in the elderly: a histopathological review of 50 cases in men aged 60 years or over. Mod Pathol 21: 54-59. [Crossref]
- Stang A, Trabert B, Wentzensen N, Cook MB, Rusner C, et al. (2012) Gonadal and extragonadal germ cell tumours in the United States, 1973-2007. Int J Androl 35: 616-625. [Crossref]
- Kaur H, Singh D, Peereboom DM (2003) Primary central nervous system germ cell tumors. Curr Treat Options Oncol 4: 491-498. [Crossref]
- Spermon JR, AWitjesa J, Kiemeney LALM (2000) Difference in stage and morphology-adjusted survival between young and elderly patients with a testicular germ cell tumor. Urology 60: 889-893.
- Wheater MJ, Manners J, Nolan L, Simmonds PD, Hayes MC, et al. (2011) The clinical features and management of testicular germ cell tumours in patients aged 60 years and older. BJU Int 108: 1794-1799. [Crossref]
- Bhardwa JM, Powles T, Berney D, Baithun S, Nargund VH, et al. (2005) Assessing the size and stage of testicular germ cell tumours: 1984-2003. BJU Int 96: 819-821. [Crossref]
- Biggs ML, Schwartz SM (2007) Cancer of the testis. In: Ries LA, editor. SEER Survival Monograph: Cancer Survival Among Adults: U.S. SEER Program, 1988-2001. Bethesda, MD: National Cancer Institute, pp : 165-170.
- Feldman DR, Voss MH, Jacobsen EP, Jia X, Suarez JA, et al. (2013) Clinical Features, Presentation, and Tolerance of Platinum-Based Chemotherapy in Germ Cell Tumor Patients. 50 Years of Age and Older. Cancer 119: 2574-2581. [Crossref]
- Loehrer PJ Sr, Johnson D, Elson P, Einhorn LH, Trump D (1995) Importance of bleomycin in favorable-prognosis disseminated germ cell tumors: an Eastern Cooperative Oncology Group trial. J Clin Oncol 13: 470-476. [Crossref]
- Bosl GJ, Geller NL, Bajorin D, Leitner SP, Yagoda A, et al. (1988) A randomized trial of etoposide+cisplatin versus vinblastine+bleomycin + cisplatin + cyclophosphamide + dactinomycin in patients with goodprognosis germ cell tumors. J Clin Oncol 6: 1231-1238. [Crossref]
- Horwich A, Oliver RT, Wilkinson PM, Mead GM, Harland SJ, et al. (2000) MRC Testicular Tumour Working Party: A medical research council randomized trial of single agent carboplatin versus etoposide and cisplatin for advanced metastatic seminoma. MRC Testicular Tumour Working Party. Br J Cancer 83: 1623-1629. [Crossref]
- Bajorin DF, Sarosdy MF, Pfister DG, Mazumdar M, Motzer RJ, et al. (1993) Randomized trial of etoposide and cisplatin versus etoposide and carboplatin in patients with good-risk germ cell tumors: a multiinstitutional study. J Clin Oncol 11: 598-606. [Crossref]
- Rentinck ME, Nieboer P, Sleijfer DT, Gietema JA, van der Graaf WT (2003) Chemotherapy for metastatic seminoma in elderly patients. Anticancer Res 23: 3093-3096. [Crossref]
- Hurria A, Togawa K, Mohile SG, Owusu C, Klepin HD, et al. (2011) Predicting chemotherapy toxicity in older adults with cancer: a prospective multicenter study. J Clin Oncol 29: 3457-3465. [Crossref]
- Extermann M, Boler I, Reich RR, Lyman GH, Brown RH, et al. (2012) Predicting the risk of chemotherapy toxicity in older patients: The Chemotherapy Risk Assessment Scale for High-Age Patients (CRASH) score. Cancer 118: 3377-3386. [Crossref]
- de Wit R, Roberts JT, Wilkinson PM, de Mulder PH, Mead GM, et al. (2001) Equivalence of three or four cycles of bleomycin, etoposide, and cisplatin chemotherapy and of a 3- or 5-day schedule in good-prognosis germ cell cancer: a randomized study of the European Organization for Research and Treatment of Cancer Genitourinary Tract Cancer Cooperative Group and the Medical Research Council. J Clin Oncol 19: 1629-1640. [Crossref]
- de Wit R, Stoter G, Sleijfer DT, Kaye SB, de Mulder PH, et al. (1995) Four cycles of BEP versus an alternating regime of PVB and BEP in patients with poor-prognosis metastatic testicular non-seminoma; a randomised study of the EORTC Genitourinary Tract Cancer Cooperative Group. Br J Cancer 71: 1311-1314.
- Nichols CR, Williams SD, Loehrer PJ, Greco FA, Crawford ED, et al. (1991) Randomized study of cisplatin dose intensity in poor-risk germ cell tumors: a Southeastern Cancer Study Group and Southwest Oncology Group protocol. J Clin Oncol 9: 1163-1172. [Crossref]
- O'Sullivan JM, Huddart RA, Norman AR, Nicholls J, Dearnaley DP, et al. (2003) Predicting the risk of bleomycin lung toxicity in patients with germ-cell tumours. Ann Oncol 14: 91-96. [Crossref]



