β2-microglobulin(β2m) is the precursor protein of the dialysis related amyloidosis (DRA) in long term hemodialysis patients.
As well approved in current experimental studies over 20years or more, the intermediate molecules, i.e., the conformational variants of globular native protein, had been confirmed in the transitional process of the in vitro amyloidogenesis [1]. The presence of this molecule in hemodialysis (HD) setting had firstly reported in amyloid tissue from a femoral bone cyst in patient with the DRA by Bellotti’s group [2]. Then, we had identified this intermediate β2microglobulin (I-β2M) using with capillary electrophoresis (C.E) in serum not only from HD patients but also the chronic kidney disease (CKD) patients and healthy persons, then, we proposed “β2M shuttle hypothesis “ as amyloidogenic concept in clinical setting of HD [3,4]. This concept is based upon 5 evidences as follows:
1) A presence of I-β2M in serum,
2) Highly amyloidogenic variant, i.e. ⊿N6β2M as well as 92-99β2M found in the amyloid tissue, not in serum.
3) Aberrant I-β2M in post HD serum which is supposed to contain a part of interstitial fluid by rebound phenomenon,
4) Implication of glycosaminoglycan (GAGs) including heparin as promoting factors and
5) An Alibi of amyloid β2M (Aβ2M) in serum.
We reviewed our concept herein again.
- I-β2M; As confirmed with wildβ2M (Figure 1), β2M consist principally of 2 molecular components on C.E, i.e. one with major population of native molecule (N-β2M) and another with minor population of intermediate molecules ( I-β2M ), in the body fluid. Proportion of N-/I-β2M in serum vary roughly from 5 to 10, but no difference can be seen among patients with CKD, HD patients and healthy persons. However, HD provoke a drastic conversion from N-β2M to I-β2M and, consequently, post HD serum at 1 hour later ,which is supposed to contain at part the interstitial β2M, showed various C.E profile among individual cases but mostly showed profile with increased proportion of I-β2M accompanied by subpopulations of more cathodicβ2M( I’-β2M) as shown in Figure 2 [4].
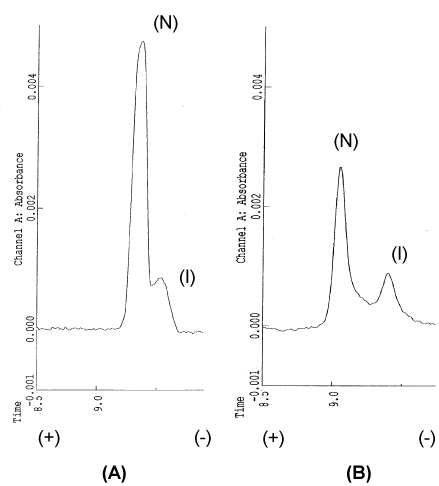
Figure 1: CE analysis of standard β2m.
(A) Standard β2m (Sigma); (B) treatment by 50% acetonitrile, peak 1: nativeβ2m(N), peak 2: intermediateβ2m(I).
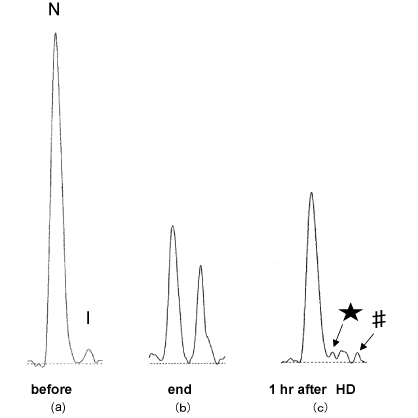
Figure 2: CE profiles.
(a) at the start and the end of HD(b), and at 1hr after HD(c). (★) a peak on refolding, (#) a peak on more unfolding. [4]
As for molecular structure, I-β2M consists of species with partially unfolded C-terminal, which can refold reversibly to N-β2M. Whereas, I’-β2M is supposed to consist of species with more, but not completely, unfolded C-terminal, which might be unlikely to refold to N-β2M.
- The unfolding of the C-terminal; The conformational variant with the unfolded C-terminal from 92Ile to 99Met had been firstly demonstrated by Stoppini et al. and we proved to be present in amyloid tissue with monoclonal antibody in 2005 [5,6]. Few years later, we had also confirmed that the C-terminal of ⊿N6β2M was completely unfolded as same as 92/99β2M [7]. In addition, we had showed “smoking gun” evidence that heparin could provoke the C-terminal unfolding in native β2M at clinical doses in HD setting, demonstrating causative implication of interstitial GAG molecules in the C-terminal unfolding of β2M because heparin is one of main GAG molecules as matrix substance in the interstitial space [8].
- ⊿N6β2M; ⊿N6β2M is a fragmental variant lacking 6 N-terminal amino acids which had been proved to be highly amyloidogenic and, therefore, be useful as model molecule for A-β2M [9]. ⊿N6β2M had firstly reported to be found in amyloid tissue from patients with the carpal tunnel syndrome and considered to be a degradation product by protease from N-β2M or I-β2M in the amyloid tissue [9,10]. The amyloidogenicity of ⊿N6β2M was directly proved by us using with the aptamer specific for ⊿N6β2M [11].
- An Alibi of A-β2M in serum; Amyloid proteins is an ultimately unfolded conformer of physiological native proteins including β2M which is a pivotal component of MHC-I and the precursor protein of this amyloidosis. Thus far, any kind of amyloid protein have not been reported in serum in any kind of amyloidosis. Similarly, as forβ2M、we had also denied a presence of both A-β2M and ⊿N6β2M in serum from HD patients with LC/MS analysis as shown in Figure 3 [12]. However, charge state ions showed interesting differences between standard β2M (Sigma) and ⊿N6β2M. Our study imply that amyloid protein must be formed in extravascular space and cannot transfer crossing vascular wall, and more importantly, cannot be cleared via the kidney or even dialysis.
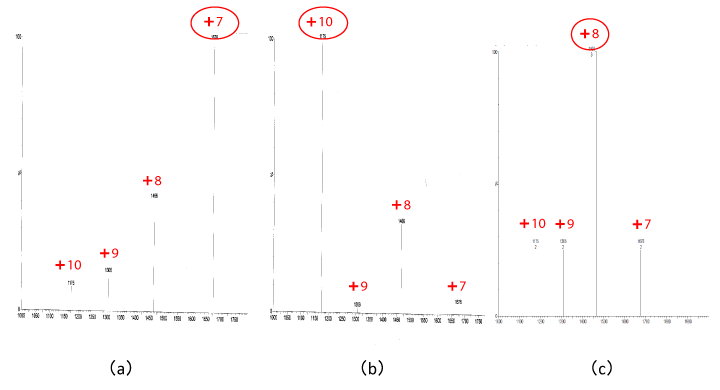
Figure 3: The m/z spectra of β2m, purified human urine β2m (Sigma) is centered at z=+7(a)
⊿N6β2m is centered at z=+10(b) and uremic serum is centered at z=+8(c). [12]
- β2M shuttle concept in development of the DRA (Figure 4); β2M, both N-β2M and I-β2M, is considered to shuttle almost freely crossing over the vascular wall and the C-terminal of I-β2M in serum might be partially, not completely, unfolded. Whereas, in the interstitial space, there co-exist 3 kinds of β2M species, i.e., N-β2M, I-β2M and β2M92-99 with completely unfolded C-terminal, which might hard to refold into N-β2M. First, HD procedure, by itself, give rise to a conversion from N-β2M to I-β2M, both of which, then, undergo more unfolding at the C-terminal in the extravascular space, i. e, space rich of GAG molecules with SO3 moiety. Next, some I-β2M species interact with GAG to convert to β2M92-99, which can give rise to polymer and some I-β2M return into the vascular space and refold again to N-β2M [4].
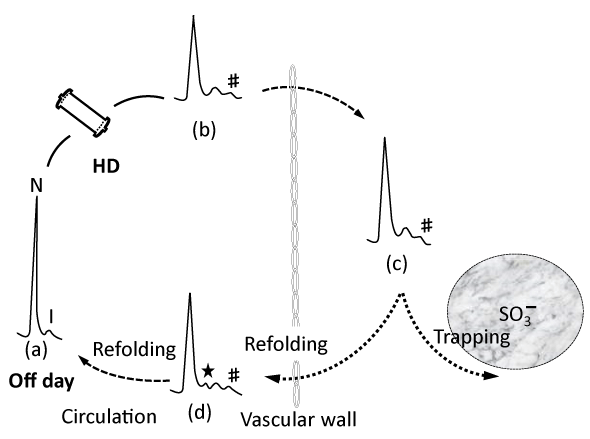
Figure 4: Illustrated dynamic shuttle of β2m. (4) (★) a peak on refolding, (#) a peak on more unfolding.
In conclusion, β2M in serum exist ubiquitously at dynamic equilibrium between N-β2M and I-β2M with overwhelming predominance of N-β2M over Iβ2M. We believe that the presence of I-β2M with the unfolded C-terminal is “sine qua non” in development of the DRA, because a C-terminal unfolding could be also confirmed in the natural A-β2M, i.e, D76Nβ2M [8,13]. HD treatment provoke inevitably a drastic shift from N-β2M to I-β2M inside vascular wall and more unfolding at the C-terminal simultaneously outside of vascular wall. In addition, along with years of HD, the C-terminal of I-β2M transferred into the interstitial space have been becoming more unfolded and resulted in accumulation of β2M92-99, which lead to straightly a development of the DRA in the matrix space.
- Chiti F, Manglone P, Andreola A et al : Detection of two patially structured species in the folding process of the amyloidogenic protein, β2-microglobulin. J Mol Biol 2001; 307: 379-391.
- Bellotti V, Stoppini M, Mangione P, et al : β2-microglobulin can be refolded into a native state from ex vivo amyloid fibrils. Eur J Biochem 1998; 258 : 61-67.
- Uji Y、Motomiya Y, Ando Y, et al: A circulating β2-microglobulin intermediate in hemodialysis patients. Nephron Clin Pract 2009; 111: c173-c181.
- Motomiya Y, Uji Y, Ando Y, et al;Capillary electrophoretic profile of β2-microglobulin intermediate associated with hemodialysis. Ther Apher Dial 2012; 16:350-354.
- Stoppini M, Bellotti V, Mangione P, et al: Use of anti β2-microglobulin mAb to study formation of amyloid fibrils. Eur J Biochem 1997; 249: 21-26.
- Motomiya Y, Ando Y, Haraoka K. et al: Studies on unfolded β2-microgulobulin at C-terminal in dialysis-related amyloidosis. Kidney Int 2005; 67: 314-320.
- Motomiya Y, Higashimoto Y, Uji Y, et al : C-terminal unfolding of an amyloidogenic βⅡ-microglobulin fragnment: ⊿N6β2-microglobulin. Amyloid 2015; 22: 54-60.
- Fukasawa K, Higashimoto Y, Motomiya Y, et al : Influence of heparin molecular size on the induction of C-terminal unfolding in β2-microglobulin: Mol Biol Res Commun 2016; 5: 225-232.
- Esposito G, Michelutti R, Verdone G, et al: Removal of the N-terminal hexapeptide from human beta2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci 2000; 9: 831-845
- Linke RP, Hampl H, Lobek H, et al: Lysine-specific cleavage of β2-microglobulin in amyloid deposits associated with hemodialysis. : Kidney Int 36: 675-681, 1989
- Fukazawa K, Higashimoto Y, Ando Y, et al: Selection of DNA aptamer that blocks the fibrillogenesis of a proteolytic amyloidogenic fragment of β2m. Ther Apher Dial.22: 61-66, 2018
- Yoneda T, Hori S, Yoshida K, et al: Analysis of serum β2 microglobulin from hemodialysis patients using liquid chromatography/mass spectrometry. J Jpn Soc Dial Ther.2019; 52: 451-455
- Valleix S, Gillmore JD, Bridoux F et al: Hereditary systemic amyloidosis due to Asp76Asn variant β2-microglobulin. N Eng J Med 2012; 366: 2276-2283.




