The four-onion peel-like predisposed ooplasms (α, β, у, δ) that play a fundamental role during the early patterning of the avian embryo giving rise to the formation of the earliest cell lineages, are already visible in the germinal discs of medium sized post-lampbrush oocytes, several days before fertilization.
After fertilization and even after oviposition these ooplasms are taken up in the blastoderm and interact with neighboring structures. So, a central β-ooplasm-containing zone develops in which the embryo proper will appear during neurogastrulation. In the wake of the germinal vesicle during its migration to the oocytal surface, elements of the Balbiani complex become localized at its deep pole forming the “endoplasmic cone” (germinal ooplasm: fifth ooplasm).
This central zone (area centralis) is surrounded by a box-like ooplasmic zone, where “extra-embryonic” ooplasms (α-, у-and δ-) play an inducing and mechanical shaping role during early patterning of the embryo. During the cleavage period, the most superficial and mobile α-ooplasm (cortex) forms, temporally a cover over the embryonic blastomeres.
The stable equatorial у-ooplasm (forming the Rauber-Koller’s sickle material) derived from the oocytal densely packed Wedge granules layer, encircles the area centralis during the formation of the hemi-circular Anlage fields (gastrulation) in the upper layer. The deep δ-ooplasm-containing endophyll functions as signalling center and induces pre-neurulation in the overlying upper layer cells of the area centralis.
The differentiation of the whole coelomo-cardiovascular system is not only dependent from the caudo-cephalic spreading and uptake of Rauber’s sickle material but also from its dorso-ventral localization (superficial caudo-central area vasculosa versus deep cranio-lateral heart tube formation).
avian blastoderm, ooplasms, neurogastrulation, coelomo-cardiovascular system
In birds, the role of the “organizer” has first been attributed to the Hensen’s node [1,2], but also the primitive streak as a whole, was considered to play an organizing role in normal development. A genuine proof that the Hensen’s node in birds is an organizer was first demonstrated by xenoplastic (duck-chicken) transplantation experiments [3-5]. A transplanted Hensen’s node evoked the development of a new neural plate in the extraembryonic area of the host blastoderm, leading to the formation of a secondary embryo. Also, HARA studied in extensu the induction of regional neural differentiation in birds [6,7]. According to Eyal-Giladi, et al. and Bachvarova et al. in pre-primitive streak embryos avian caudal marginal zone cells function as primitive streak inducers [8,9]. However, the caudal marginal zone in their description seems more or less, without precision, to be composed of different components. Among which we demonstrated that the Rauber-Koller’s sickle material is the real primary gastrulation organizer of the avian blastoderm [10]. Moreover, gastrulation and neurulation are not impaired by removal of the marginal zone [11-13]. This article was written with the aim to study the gradient-like centrally directed uptake of different ooplasms in the avian blastoderm giving rise to the caudo-cephalic orientation of the embryo. Thereafter by studying the mutual local interactions of the predisposed ooplasms, the patterning of the embryo could be explained (inductory and mechanically). Moreover, the formation of the coelomo-cardiovascular system depends on the spatial distribution of Rauber’s sickle material [14-18].
By careful isolation of quail Rauber’s sickle [19,20,21] material present in the caudal half of the blastoderm and culture in vitro of quail–chicken chimeras, we clearly demonstrated that more precisely the Rauber’s-Koller’s sickle material and not the caudal marginal zone (in which the area vasculosa caudalis develops: [22,23] is the primary, major organizer of the avian unincubated blastoderm [10,24]. According to Izpisua et al. [25] the chick homeobox gene goosecoid (gsc) is first expressed in Rauber’s-Koller’s sickle material of unincubated chicken blastoderms, which indicates the presence of organizer cells in this structure. Rauber-Koller’s sickle seems to be homologous to the Nieuwkoop’s center [26] in amphibia [24].
Usually the ooplasm below the avian blastoderm is considered as mainly formed by inactive yolk (deutoplasm).
For morphological and experimental studies with avian blasdoderms, usually the unincubated blastoderms are “stripped” off from their underlying egg yolk ball, which mostly results in the loss of some structural aspects and the real relationships between ooplasms and neighboring blastoderm parts.
To avoid this during our observations, this histological fixation of the avian blastoderm was performed in situ on its egg yolk ball immersed in the fixative. So, the intact relationship between the blastoderm parts and neighboring ooplasms was conserved.
This was f.i. important for the intact conservation of Rauber’s sickle (RS) and neighboring structures which until now were not correctly observed: the axilla, the sickle canal, the sickle endoblast and the so-called “nodus posterior”, the caudal part of the primitive streak (PS). Also, the uptake of distinct labeled ooplasmic material could so been established. Also, the gravitational influence of the rotating egg yolk ball mass on the development of the gradient-like caudo-cephalic axis and ensuing coelomo-cardiovascular system can so be followed.
In the unincubated avian blastoderm (Figures. 1A, 1B) we discerned three stem cell lineages (elementary tissues): the so-called upper layer or ectophyll forms the surface of the whole embryo (over the area centralis, area pellucida and marginal zones) and covers the distinct deep layer components: the caudal marginal zone, the caudal Rauber’s sickle (RS) and the more centrally localized thinner endophyll (primary endoblast), localized in the planar prolongation of the Rauber’s sickle: the endophyll (primary endoblast). In response to signals from the endophyll the cranial neural plate Anlage (pre-neurulation), expresses Otx-2 and plays a role for orientation and organization of the early head region [27,28].
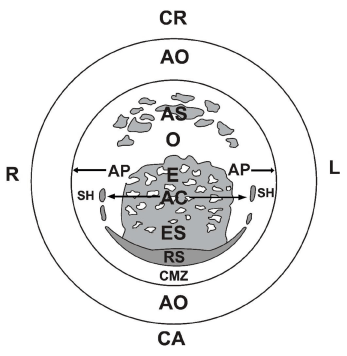
Figure 1A. Schematic representation of the different components visible from the deep side of a laid unincubated chicken blastoderm:
R: right and L: left side; CR (cranial) and CA (caudal region); RS: median-part of Rauber- Koller’s sickle, the earliest organizer; SH: sickle horns; E: endophyll; ES: endophyll in which sickle endoblast derived from RS has migrated cranially; AS: anti-sickle region formed by upper layer (UL) in which no RS material is present, but remnants of yolk masses or cell groups; O: region where only the deep side of the UL is visible; AC: area centralis in which neuro-gastrulation phenomena take place, encircled by median RS and lateral SH; AP: area pellucida (borders indicated by horizontal arrows); CMZ: caudal marginal zone in which after incubation blood islands (area vasculosa caudalis) will develop; AO: area opaca.
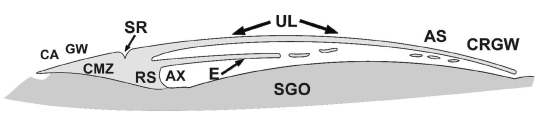
Figure 1B. Schematic medio-sagittal section through an unincubated chicken blastoderm after fixation in situ and in toto on the egg yolk ball. Note that the caudal marginal zone (CMZ) and Rauber’s sickle (RS) are in permanent contact with the caudal subgerminal ooplasm; in the anti-sickle region (AS) and below the cranial germ wall (CRGW) this is not the case since there an open narrow space exists; SR: “Sichel Rinne”; AX: axilla, caudal part of the subgerminal space, enclosed between the blastoderm and the subgerminal ooplasm (SGO).
Diametrically opposed to the Rauber’s sickle region we see the anti-sickle (AS) region in which isolated fragments of deep layer cell-groups or yolk masses are localized close to the neighboring upper layer (UL). In the anti-sickle region, the upper layer (UL) extends loosely over the underlying yolk mass. In the caudal Rauber sickle region, the Rauber sickle (RS) is fixed in continuity to the underlying subgerminal у-ooplasm, forming an axilla-shaped (Axilla) part of the subgerminal cavity, but also the Rauber sickle region adheres to the overlying upper layer (UL) usually forming a superficial sulcus [21] (Figure. 1B). This adhesion can have been demonstrated by radioactive labeling of the y-ooplasm (Figure. 4A).
Temporo-spatial disposition of the structures in the avian blastoderm was visualized after successive (usually daily) radioactive labeling followed by autoradiography [29] or by the trypan-blue-induced fluorescence method [30-32]. By these methods it is possible to follow the evolution of the peripherally assembled yolk layers not only in the germ disc but also in their prolongation in the neighboring yolk mass. This indicates, contrary to what was usually assumed [33], that the germ disc or blastoderm not floats separately on the top of the yolk mass but that it forms an integral part of it [29]. This permitted us to discern the localization of the four ooplasms (giving rise to four cell lineages) with reference to the labeled layers [14] in the germ disc during the post-lampbrush stage (Figure. 2A), several days before fertilization.
So we could demonstrate the pre-existence of onion peel-like ooplasms (α-, у-, δ-) forming an ooplasmic “box”, surrounding the central β-ooplasm in which the first embryonic structures will appear. These ooplasms are wholly different from the classically described germ layers, originally described by Pander and Von Baer which are only derived from the β-ooplasm [34,35].
The α-ooplasm is localized in the oocytal surface region (Figure. 2A) and plays an important role during the cleavage stage (Figure. 2B). During the early chicken cleavage stages a chicken vasa homolog (CVH) positive granulo-fibrillar structure (approximately 20 µm in diameter) was found in the basal part of the cleavage furrows [36]. This is the region where the α-ooplasm penetrates into the nucleus of Pander and even becomes localized below the subgerminal cavity [16,23,32,37-39]. This is in agreement with our observation after trypan-blue-induced fluorescence labeling that the primordial germ cell material is originally localized in the depth of the germ disc [40] at the level of the superficial part of the nucleus of Pander [32,39,41]. This also clearly demonstrates that primordial germ cells are not derived from the epiblast as was claimed by Eyal-Giladi et al. [42]. The very mobile α-ooplasm belongs to the ooplasmic surface, which may possess properties which (in the amphibian grey crescent) are inherited directly from one generation to the next without the intervention of classical genes [43,44]. Curtis concluded for self-replicating properties of part of the cortex.
The β-ooplasm (Figures. 2A, 2B) contains the interrupted layer of the t.i.c.o.s (ᶟH-thymidine incorporating, RNA-rich organelles, containing mitochondria) [37]. The β-ooplasm layer mainly forms the embryo proper, enclosed as in an “ooplasmic box” by the surrounding α-, у- and δ-ooplasm-containing structures. The у-ooplasm is equator-likely disposed and composed of densely packed yolk granules forming the broad Wedge granules layer in the oocytes (Figure. 2A), after penetration of the peripherally assembled yolk through the more superficial α- and β-ooplasms (Figure. 2C) [45]. By the presence and later disappearance of the voluminous germinal vesicle, the dense Wedge granules layer is centrically interrupted and forms an annular structure (containing y-ooplasm). After fertilization and symmetrization (Figures. 1B, 2B) the Rauber’s sickle is formed by an one-side uptake of Wedge granules layer-material (у-ooplasm) in the future caudal part of the blastoderm [23]. By deep oocytal labeling of part of the nucleus of Pander (containing primordial yolk spheres: δ-ooplasm), 6-7 days before fertilization (Figure. 2D) the endophyll and the ooplasm of the PG Cells (germinal yolk) in the embryo can be labeled [30,32].
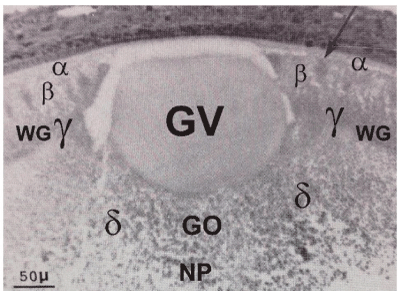
Figure 2A. Low power view of the germinal vesicle (GV) and the four surrounding ooplasms (α-,β-,у-,δ-) in a quail oocyte of 5,5 mm diameter; t.i.c.o’s (indicated by arrow) are seen in the β-ooplasm; у-ooplasm localized in the Wedge granules layer (WG); δ-ooplasm is seen in the superficial part of nucleus of Pander (NP); the presumptive germinal ooplasm (GO) giving rise to ooplasm in the PG Cells is localized in the central δ-ooplasm of the nucleus of Pander, below the deep pole of the germinal vesicle (endoplasmic cone probably derived from the Balbiani complex material).
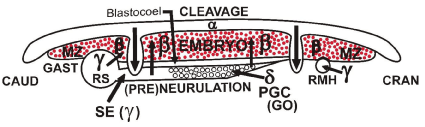
Figure 2B. Schematic drawing of a section through the avian embryo (β-ooplasm) in an “ooplasmic box” model presenting the phenomena of cleavage, gastrulation and (pre)neurulation induced, guided and shaped by the surrounding “extra embryonic” α-,у-,δ- ooplasms-containing structures, forming a flat “box” around the embryo proper: the α-ooplasm is found in the roof of the “box” [23]
the у-ooplasm, in the form of an equatorial ring, forms the walls of the “box”,
the δ-ooplasm and later the cranially growing sickle endoblast (SE: у-ooplasm) derived from the Rauber’s sickle form the bottom of the “box”.
PGC: primordial germ cells derived from the germinal ooplasm (GO).
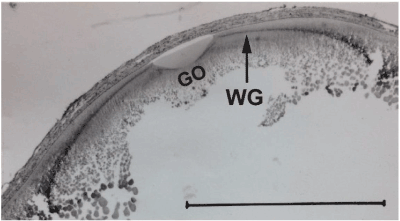
Figure 2C. Autoradiography of a section through the germinal disc region of a quail oocyte (14 mm diameter), 3 days after an injection of ᶟH-leucine to the mother; the arrow indicates labeled yolk in the Wedge granules layer(WG); GO: presumptive germinal ooplasm region localized below the deep pole of the germinal vesicle in the nucleus of Pander, encircled and domed by the Wedge granules layer. Bar = 1mm. [45]
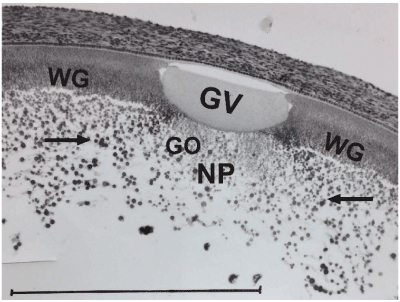
Figure 2D. Autoradiography of a section through the germinal disc region of a 6 mm-diameter quail three days after a maternal injection of ³H-leucine: GV: germinal vesicle with central post-lampbrush chromosomes; NP: nucleus van Pander; WG: Wedge granules; GO: presumptive localization of germinal ooplasm with elements of the Balbiani complex, surrounded by a sphere of labeled primordial yolk (arrows) at the deep pole in the wake of the germinal vesicle; the diameter of the radioactively labeled yolk sphere (approximately 500 μm-diameter) indicates that the oocyte at the moment of maternal injection was in the lampbrush stage. Bar = 500 μm.
First described by Balbiani [46] in myriapods and spiders, this paranuclear ooplasmic structure is very prominent in the prelampbrush oocytes of birds, where it finally forms a central fine meshy network in older oocytes [47-49]. The spherical oocytal radioactive labeling (diameter proximately 500 µm) after ³H-leucine application, seen in Figure 2D indicates that the Balbiani complex was still in paranuclear deep contact with the germinal vesicle at the lampbrush stage (approximately 500 µm diameter) and remains so during the ensuing post-lampbrush stage. This was also suggested by the histochemical staining of the Balbiani body after cobalt nitrate fixation [41].
In Xenopus this also could be derived from the remnants of the Balbiani complex body [50] in which a message transport organizer (METRO) has been found, containing germinal plasm [51,52]. At the deep pole of the germinal vesicle during early development of quail oocytes in post-lampbrush stage we observed a so-called “endoplasmic cone [53]. This corresponds to the part of germinal yolk plasm (fifth ooplasm) in the deepest central part of the germ disc (Figures. 2A, 2C, 2D) which settles finally in the PG Cells of the next generation [32,41] and seems also derived from Balbiani complex material. A germline-specific-expression of chicken vasa homolog protein (homolog to the vasa gene which plays an essential role in germline formation in Drosophila) has been used for tracing the origin of avian primordial germ cells [36]. In chicken oocytes, they found that CVH protein was predominantly localized in granulo-fibrillar structures surrounding the mitochondrial cloud and spectrin proteinenriched structure, indicating that the CVH-containing ooplasmic structure is the precursory germ plasm in birds.
By this method the germline-specific-expression was first found close to the depth of the central cleavage furrows which extends into the δ-ooplasm of the nucleus of Pander. This corresponds to the region where we have localized the germ cell yolk, which finally settles into the primordial germ cells [32,39] (Figures. 2A, 2C, 2D).
In Anura the “germ plasm” has been localized in the depth of the vegetal hemisphere [54], which again suggests a similarity with birds.
By studies with trypan-blue induced fluorescence it was seen that a massive part of the subgerminal ooplasm was taken up in the caudal region of the area centralis of the unincubated blastoderm (Figure. 3A). Also after radioactive labeling with ᶟH-leucine of the subgerminal y-ooplasm we could see the uptake of large labeled islands of this ooplasm by the encircling movements of more superficial cellular material of the RS (Figures. 3B, 3C). The border between celluralized and not celluralized ooplasm is thus not always clearly defined. From both types of ooplasm induction phenomena on neighboring embryonic tissues can occur, since transition phenomena between both types exist. This shows that during early development of the avian blastoderm (still at the end of the intrauterine period) and after early incubation subgerminal ooplasm is taken up, indicating that both structures were not completely separated and still interact during beginning incubation. This can clearly been seen during the formation of the massive junctional endoblast, at early incubation. So, we observed that both embryonic and/or extra cellular material from the previous oocytal generation has a gradient-like influence on the caudo-cranial and coelomo-cardiovascular development of the present embryo.
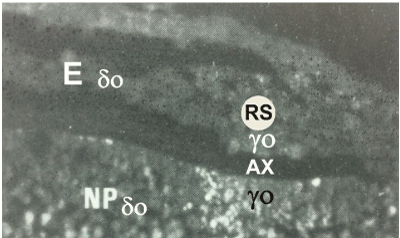
Figure 3A. Trypan-blue induced fluorescence in a medio-sagittal section through the caudal part of an unincubated quail blastoderm, after a maternal injection with trypan-blue solution, four days before. The uptake of trypan-blue labeled subgerminal ooplasm (у-ooplasm: yo) into the overlying Rauber’s sickle (RS) region, separated by the axilla (AX), is clearly seen. Fluorescent yolk granules are found in the prolongation of the underlying fluorescent subgerminal у-ooplasm indicating direct uptake of the у-ooplasm into the caudal part of the blastoderm (RS), without massive peripheral or caudo-cephalic cellular displacement. E: endophyll and primordial germ cells (δ-ooplasm: ± δo) taken up from the nucleus of Pander (NP) are not labeled.
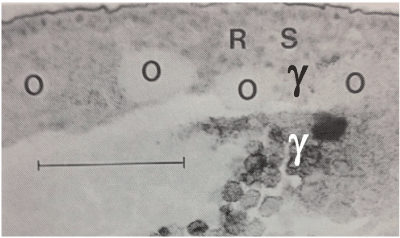
Figure 3B. Autoradiograph of a medio-sagittal section through the caudal region of an unincubated quail blastoderm, laid four days after a maternal injection of ᶟH-tyrosine.
O: islands of labelled subgerminal ooplasm (y-) are taken up in the deep caudal part of the blastoderm by encircling and surrounding cell masses. Scale bar = 100 μm.
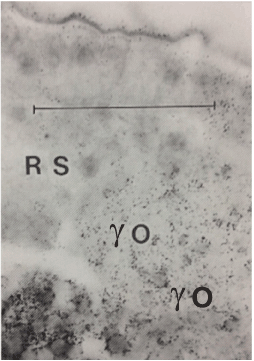
Figure 3C. Higher magnification of part of the preceding autoradiograph, showing the direct massive uptake in the Rauber’s sickle without pronounced lysis of the ooplasm. The labeled yolk and the surrounding intervitelline ooplasm is still visible in the “island” (yo). Scale line = 50 μm.
From a comparative literature study Eyal-Giladi [55], concludes that the establishment of the axis in chordates (axialization) with bilateral symmetrization, depends on the translocation of oocytal (maternal) determinants from the vegetal pole toward the future dorso-caudal side of the embryo. On their arrival at their destination, the activated determinants form in all chordates an induction center, homologous to the Amphibian “Nieuwkoop center” (or avian Rauber’s sickle), which later will induce, respectively, the formation of the Spemann’s organizer or the avian Hensen’s node. In the case of birds these determinants seem to be originally present in the deep peripheral equator-like circular y-ooplasm derived from the wedge granules layer, doming and surrounding the δ-ooplasm of the nucleus of Pander. We assume that the underlying yolk-mass presses, by uni-focal mechanical pressure during the in utero rotation, part of this y-ooplasm against the deep peripheral cells of the rim of the area centralis so forming Rauber’s sickle [56,57].
The Rauber’s sickle transforms progressively in a broader structure (junctional endoblast) which has a similar aspect and also a strong embryo-inducing effect on the upper layer with dominating potencies after transplantation in heterotopic regions on the upper layer and culture [58]. In this region, “eroded” Rauber’s sickle material is seen in close association with induced blood islands and coelomic vesicles [22,59-61], later forming the coelomo-vascular system in the yolk sac wall [17,18]. The organizing effects of the Rauber’s sickle are more extensive than those of Hensen’s node, since the Rauber’s sickle material is also indispensable for the formation of the coelomo-cardiovascular system [59,60,62]. The spontaneous oblique formation of the subgerminal space appearing between the blastoderm and the remainder of the yolk mass, together with the boot-shaped distortion of the nucleus of Pander, was first demonstrated by radioactive labeling (Figure. 4A) and later by trypan-blue induced fluorescence of the y- or δ-ooplasmic layers [31,39,56,57]. Also, the caudal adhesion of the labeled у-ooplasm and the overlying upper layer, forming a peripheral sulcus (called “Sichel Rinne” by Koller is seen (Figure. 4A) [21]. This explains, by gravitational interference between blastoderm and associated yolk mass [56], the gradient-like caudo-cephalic uptake of Rauber’s sickle material (y-ooplasm) and endophyll (δ-ooplasm) occurring respectively during gastrulation and (pre)neurulation (Figure. 4B). The gradient-like caudo-cephalic spreading of Rauber’s sickle material, encircling the area centralis provokes by induction and shaping a strong centro-caudal sliding movement (polonaise movement) of the upper layer cells in the concavity of the Rauber’s sickle giving rise in both halves (left and right) of the blastoderm to the formation of a hemi-primitive streak in the middle region [14,16, 17,63] (Figures. 5A, 5B).
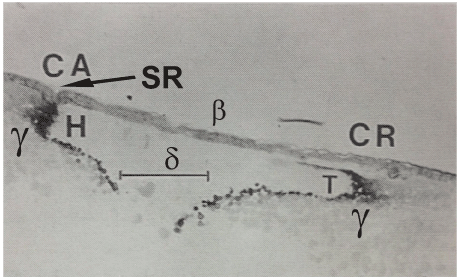
Figure 4A. Autoradiograph of a medio-sagittal section through an unincubated quail blastoderm, laid five days after a maternal injection of ᶟH- tyrosine. The separation of the cranial part (CR) of the blastoderm from the underlying hook-shaped labeled y-ooplasm (T) is clearly seen, whilst in the caudal part we see the adhesion of the labeled у-ooplasm to the neighboring part of the upper layer, forming a peripheral sulcus, SR (called “Sichel Rinne” by Koller [21] in the Rauber’s sickle region, where later the caudal area vasculosa will develop very superficially below the upper layer. In contrast, in the more cranial region the heart tubes will develop in the deeper part of the blastoderm, containing labeled у-ooplasm (sickle horns). This local different distribution of the y-ooplasm seems to predetermine the local differentiation of the coelomo-cardiovascular system.
Scale bar = 300 μm.
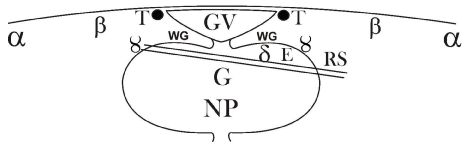
Figure 4B. Combined schematic drawing of a medio-sagittal section through a quail post-lampbrush oocyte compared with the derived blastoderm after symmetrization: the oblique split represents the spatially unequal formation of the subgerminal space resulting in the spatial oblique uptake in the deeper caudal half of the blastoderm of у-ooplasm (RS) and δ-ooplasm, forming the endophyll (E) in the future caudal region of the blastoderm. The oblique formation of the split explains why RS and endophyll are fixed together in one plane. G: presumptive localization of primordial germ ooplasm.
A good example of the fundamental importance of the interaction of y- with β-ooplasm-containing cell lineages is seen by our observation that in avian blastoderms mosaic versus regulation development, depends on the spatial distribution of Rauber’s sickle material (y-ooplasm) with reference to the upper layer (β-ooplasm) [16,17]. We have already described the effect of gravity on the interaction between the avian germ rim cells (before symmetrization) and the neighboring deeper ooplasm in partially or totally inverted egg yolk balls. After in toto culturing in egg white of such inverted egg yolk balls [64], there is a shifting and compression of the deep subgerminal ooplasm with the more superficial and peripheral blastoderm layer. On sections it is possible to see an upward ingrowth of cells, with numerous extensions into the ooplasm. This is also seen to occur during the normal formation of the Rauber’s sickle [57,58]. At the level of Rauber’s sickle, the upper layer does not appear as a uniform cell layer, but rather as a syncytium with poorly delineated cell borders, characterized by local adhesion or the mixing of the superficial and deep layers [65,66] suppose that Rauber’s sickle is originally formed by invasion of the overlying upper layer cells into the deep layer (thus creating local interaction between β-ooplasm and у-ooplasm). There is also evidence for an active Wnt-catenin pathway in the vegetal cells in the periphery of the multicellular embryo [67]. By the time of the egg laying, β-catenin has been localized in the nuclei in the peripheral zone of the blastoderm [68].
In the case of birds, determinants seem to be originally present in the deep, peripheral, equator-like and circular у-ooplasm (Wedge granules layer), which surrounds and domes the δ-ooplasm of the nucleus of Pander. We assume that the underlying yolk mass presses, by unifocal mechanical pressure created during the in utero rotation, part of this у-ooplasm against the deep peripheral cells of the rim of the area centralis, so forming Rauber’s sickle.
The formation and localization of the coelomo-cardiovascular system is directly related to the oblique formation of the subgerminal space and the oblique uptake of the Rauber’s sickle (RS) material in the blastoderm (Figures. 4A, 4B).
The localization of blood islands and associated coelomic vesicles with reference to the hemi-PS’s and migration via hemi-mesoblast mantles over the sickle canals in the caudal part of the area centralis, are represented in Figure. 5A, 5B). After у-ooplasm labeling by maternal injection of ᶟH-leucine, it is seen that the labeling in the caudal Rauber’s sickle region of the blastoderm (Sichel-Rinne: Figure. 4A) is very close to/or adhering to the surface of the blastoderm.
In contrast in the future cranial region towards the anti-sickle region the same labeled (у-ooplasm) layer has a deeper localization with reference to the surface of the blastoderm (Figure. 4A). This seems to be due to the tilting in a caudal direction of the nucleus of Pander and surrounding subgerminal y-ooplasm below the surface of the blastoderm during early symmetrization [56,57].
So there is not alone a caudo-cephalic gradient of uptake of RS material (у-ooplasm), but also the Rauber sickle horns become localized in a relatively deeper position. The difference in the depth (caudally versus cranially) of the localization of the inducing Rauber’s sickle material can perhaps explain the existence of two coelomo-vascular foci formed via two different migrating paths: one in the roof over the sickle canal (Figures. 5B, 5C) and the other through the intraembryonic cavity (Figures. 5C, 5D). There exist indeed two united systems (forming a large sickle) developed in close contact with a similar Rauber sickle (RS) or junctional endoblast material (Figures 5B, 5C, 5D, 5E).
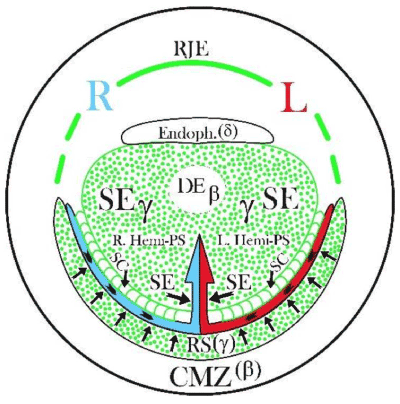
Figure 5A. Schematic representation (seen from the deep side of the blastoderm) of two important successive steps taking place under influence of two у-ooplasm-containing cell groups during the formation of a PS: 1° inductive and mechanical shaping influence by the left and right Rauber’s sickle (RS) halves (indicated by centrally directed arrows) on the encircled area centralis, forcing the influenced UL cells into a centro-caudal direction, (“polonaise” movement, indicated by arrowheads) in the concavity of Rauber’s sickle. 2° followed by the inductive influence (indicated by two large horizontal arrows) of the central sickle endoblast (SE) on the formation of two parallel median hemi-PS’s; SC: sickle canal formed by sickle endoblast localized between the central sickle endoblast and the Rauber’s sickle; R: right and L: left side of the blastoderm; RJE: rostral junctional endoblast in the prolongation of the RS, forming the rostral limit of the area centralis; CMZ: caudal marginal zone containing β-ooplasm in the upper layer (UL), since here also an embryo can be induced by transplanted sickle endoblast or endophyll (δ-ooplasm); normally the endophyll is pushed in a cranial direction (endophyllic crescent) by the sickle endoblast.
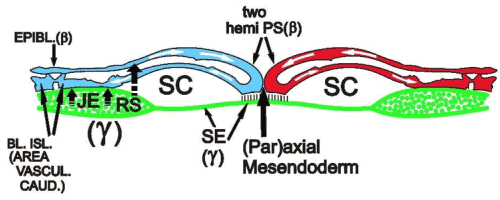
Figure 5B. Interaction between у- (green colour) and β-ooplasm-containing cell lineages, during gastrulation and caudal area vasculosa formation: schematic representation of a section through the caudal part of Figure 5A, showing the sliding (indicated by white arrows in the median region) forming two parallel hemi-PS’s (left and right) which will migrate laterally in the roof of the sickle canal (SC) to form blood islands and coelomic vesicles in the neighborhood of the junctional endoblast (JE); two short vertically directed arrows indicate the inducing effect of the junctional endoblast (JE) on blood island formation (BL.ISL); long the interrupted arrow, starting from the RS and pointing to the epiblast (UL), indicates the start of the induction of mesoblast by Rauber’s sickle; SE: sickle endoblast, which induces the (par)axial mesendoderm.
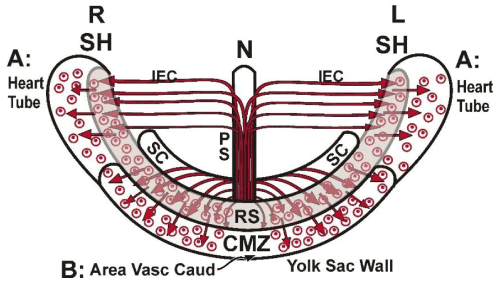
Figure 5C. Schematic representation (ventral view) of the formation of the two regions (A and B) of the early avian coelomo-cardiovascular system, (forming an entirety) represented as small circles (coelomic vesicles) surrounding dots (blood islands):
A: the bilateral primary heart tubes and pericard precursor cells, derived from the cranial part of the PS, migrating via the intraembryonic cavity (IEC) come in contact with the inducing sickle horn material.
B: the area vasculosa caudalis, formed by the caudal mesoblast cells, migrating laterally in the roof of the sickle canal and over the inducing RS (junctional endoblast) into the caudal marginal zone.
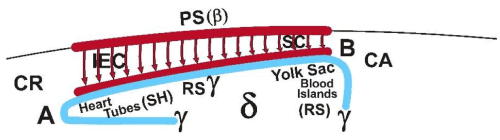
Figure 5D. Schematic para-sagittal representation (seen from medially) of the different development of the two regions (A and B) of the coelomo-cardiovascular system (forming an entirety) under the influence of the neighboring caudo-cephalic and dorso-ventral spreading of the Rauber’s sickle material (у-ooplasm). The heart tubes develop cranially (CR) and more deeply. The area vasculosa caudalis (yolk sac) develops caudally (CA) and more superficially (yolk sac).
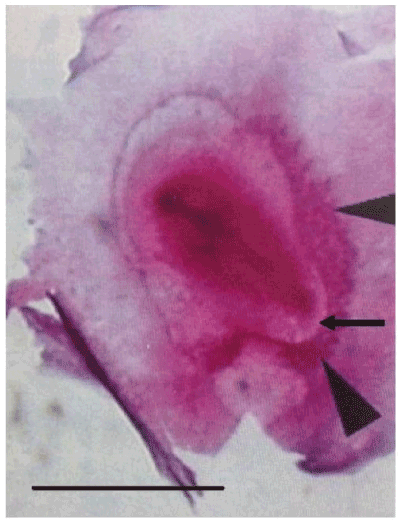
Figure 5E. Demonstration of the spatial continuity of the right lateral area vasculosa (lateral arrow head) and the caudal area vasculosa (caudal arrow head) in direct relationship with the spreading of the Rauber’s sickle material, by in toto staining with Unna of an avian blastoderm after 28 h of culture, from which at the unincubated stage the left sickle horn region was taken off; the arrow indicates the transparent sickle canal roof in the concavity of the caudal area vasculosa.
A. A cranially directed bilateral and deeply localized blood pumping system, forming the primary heart tubes in close association with the Rauber sickle horns [22]. It has been shown by various workers [69-71] that the presumptive heart mesoderm in the chick lies, as two separate areas on either side of the head process. The movement of the presumptive heart cells into their correct situation and ordering has been described by De Haan by the marking with particles of iron oxide of the undifferentiated splanchnic mesoderm [72-74]. Also Lopez-Sanchez et al. found that the primary heart tubes are derived from mesoblast cells ingressing according to a cranio-caudal order through the cranial part of the primitive streak [75]. There upon, according to De Haan and Bellairs, the heart and pericard precursor cells migrate through the intraembryonic cavity at a distance from the epiblast [74,76]. De Haan also describes that clusters of precardiac cells migrate more actively over an endodermal substrate which seems to correspond to the junctional endoblast (RS horns), we described here (Figures 5C, 5D).
B. A caudal median superficial blood circulation system, forming the area vasculosa caudalis in the caudal marginal zone [22], parallel with the sickle canal (Figure. 5E), giving rise to the yolk sac circulation where respiration via the superficial coelomic vesicles takes place. Nutrition of the embryo, on the contrary occurs via the deeper blood islands, close to the yolk sac endoblast [60,15]. A molecular biological difference between both systems has also been described: sizzled and vascular endothelial growth factor receptor 2 (VEGFR2) are co-expressed in caudal mesodermal hemangioblastic cells [77].
Outside this region no co-expression is observable: sizzled is expressed in the procardiac region and absent from embryonic or yolk sac VEGFR2 angioblast. VEGF is currently considered as the major inducer of angiogenesis in both normal and pathological conditions.
Evolution of the early ooplasm-dependent cell lineages during early incubation of the blastoderm. By isolation and/or combination experiments in vitro of the different elementary blastoderm tissues formed by different ooplasm-mediated cell groups (cell lineages), we were able to demonstrate their role during further development of these different parts (upper layer, Rauber’s sickle, endophyll) in the avian blastoderm.
A. In a first group of experiments, the evolution of isolated stem cell lineages or ooplasms was examined:
1°. After the culture of isolated endophyll (δ-ooplasm containing) fragments placed on a vitelline membrane according to New [78], no embryo developed, only primordial germ cells were seen in the neighbourhood of the endophyll [79,80] (Figure. 6A).
2°. After the culture of an anti-sickle (exclusively formed by upper layer cells, β-ooplasm containing) no embryo developed, only the upper layer cells transformed in a very expanding epiblast [81] (Figure. 6B).
3°. After culture of a fragment of Rauber’s sickle (y-ooplasm containing) no embryo developed, only the Rauber’s sickle fragment transformed in a flat structure extending over the supporting vitelline membrane (Figure. 6C) [10].
4°. After placing on a vitelline membrane, a central subgerminal ooplasmic mass, containing centrally nucleus of Pander-material (δ-ooplasm) and more peripherally surrounding y-ooplasm, no embryo developed (Figure. 6D).
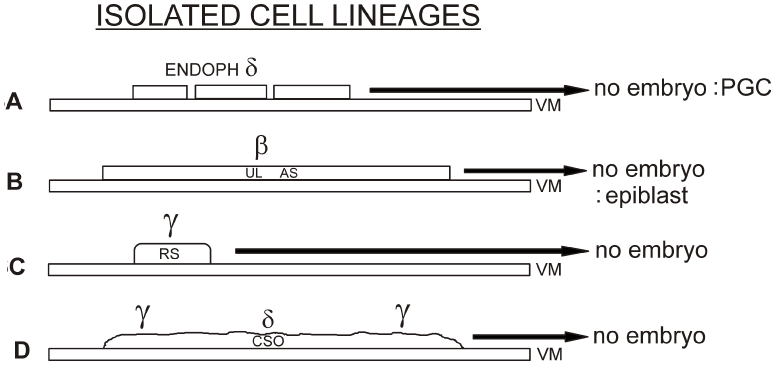
Figure 6A. After culture on a vitelline membrane (VM) of an endophyll fragment (δ-ooplasm containing), no embryo developed, only some primordial germ cells (PGC) leaving the endophyll were seen [80].
Figure 6B. After culture of an isolated fragment of the upper layer (UL) of an anti-sickle (AS) region, containing β-ooplasm, a rapidly expanding epiblast developed, but no whole embryo [81].
Figure 6C. After culture of a fragment of isolated Rauber’s sickle material (containing у-ooplasm) no embryo develops [58].
Figure 6D. After culture on a vitelline membrane (VM) of a central subgerminal ooplasmic mass, containing δ-ooplasm surrounded by equatorial у-ooplasm, no embryo develops.
B. In a second experimental group, the effect of Rauber’s sickle material on the upper layer of the blastoderm was established. A graft of quail Rauber’s sickle material placed on the deep part of the upper layer of the anti-sickle of a whole chick unincubated blastoderm, provoked the development of an induced embryo with upper layer-derived chicken cells (epiblast, neuroblast, mesoblast, blood) and quail-derived deep layer cells (junctional and sickle endoblast) (Figure. 7A). In some cases, one of the two Rauber’s sickles (the autochthonous or the grafted) disappeared by competitive inhibition since they contain the same y-ooplasm [82]. Indeed, to avoid this phenomenon, the grafting of a quail Rauber’s sickle fragment was performed on the deep side of the upper layer of an isolated chicken anti-sickle (Figure. 7B). In these cases, always, an embryo developed with a centripetally or centrifugally directed primitive streak. Here also a coelomo-vascular system developed.
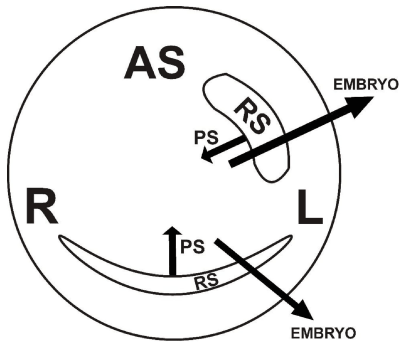
Figure 7A. Grafting a quail Rauber’s sickle fragment on the anti-sickle region (AS) of an unincubated chicken blastoderm in culture, usually evokes the formation of a secondary embryo formed of chicken cells derived from the upper layer (β-ooplasm). The inducing deep layer is formed of quail cells derived from the grafted у-ooplasm containing Rauber’s sickle material (centripetally migrating sickle endoblast and in situ proliferating V-shaped junctional endoblast). In some cases, there exists competitive inhibition between the autochthonous and grafted Rauber’s sickle material (probably to inhibit poly-embryony) and one of the two Rauber’s sickles and corresponding PS disappears [82].
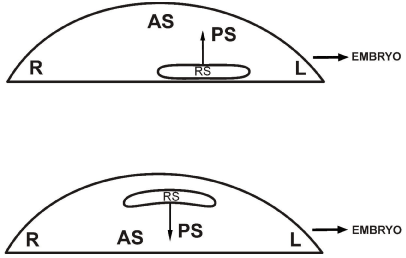
Figure 7B. After grafting a quail Rauber’s sickle fragment on an isolated chicken anti-sickle region (AS) in culture, always a miniature but complete embryo is induced with centrifugally or centripetally directed chicken primitive streak (PS) and with Rauber’s sickle derived quail sickle endoblast and quail junctional endoblast, with coelomo-cardiovascular development.
C. Role of the endophyll layer (δ-ooplasm containing). The experiments were performed in culture with an isolated caudal blastoderm quadrant.
In a caudal blastoderm quadrant, as such, the three cell lineages are present, and after culture a normal primitive streak and embryo develops in the median part of the quadrants (Figure. 8A) [81]. If the endophyll layer is completely removed in such a quadrant, no primitive streak and no embryo develop (Figure. 8B).
If however the chicken endophyll was replaced by quail endophyll, the gastrulation and neurulation potencies were restored (Figure. 8C) [81]. A normal primitive streak only developed in the upper layer of the caudal quadrant, when both endophyll and Rauber’s sickle were present. Thus endophyll cannot regenerate neither from the upper layer, nor from Rauber’s sickle [81].
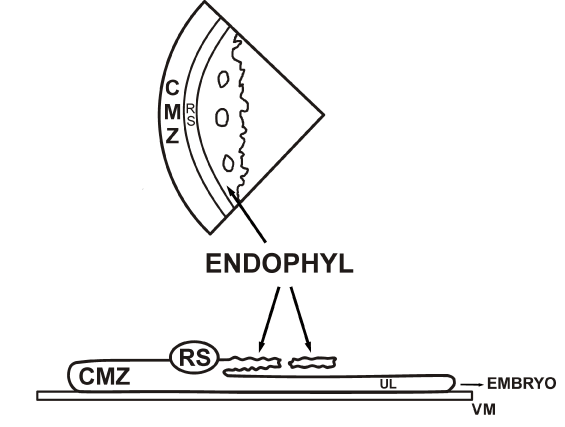
Figure 8A. After culture, in vitro of an isolated caudal quadrant of a chicken unincubated blastoderm a median normal miniature embryo develops.

Figure 8B. If in culture, the endophyll layer is cut off from the Rauber’s sickle and removed, no embryo develops, demonstrating the starter role of endophyll [81].
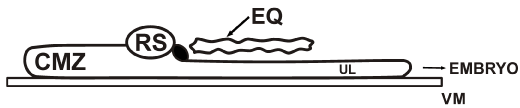
Figure 8C. If in culture the chicken endophyll layer is removed but replaced by a quail endophyll layer (EQ) a normal miniature embryo will develop.
In conclusion, our experiments indicate that if the three specific ooplasm-mediated embryonic stem cell lineages: upper layer (β-ooplasm containing), Rauber’s sickle (у-ooplasm containing) and endophyll (δ-ooplasm containing), are present at the right place and time, a normal embryonic development takes place, no regulation nor regeneration occurs when only one or two stem cell lineages are present.
We have shown by radioactive labeling and quail-chicken combination experiments [83] that the sickle endoblast has not only a mechanical influence on the endophyll by pushing it in a cranial direction [79,80], but also that it penetrates centripetally into the endophyll. Its rapid extension possibilities seem to be in agreement with the observation of Sanders et al. that in hanging drop cultures, the sickle endoblast produces large ruffling membranes and moves rapidly, with a tendency to break away from its original location [84].
D. Effect of central subgerminal ooplasm.
Figure 9 shows, a comparative schematic drawing of tissue associations, which in earlier experiments, were followed or not by embryonic development. It is only in the presence of upper layer and Rauber’s sickle that an embryo is formed in the presence of subgerminal ooplasm, so that in particular cases the central subgerminal ooplasm can be considered as an elementary tissue. This association of germinal disc components (upper layer, Rauber’s sickle and subgerminal ooplasm) followed by the formation of a caudo-cranially directed embryonic axis is found in natural conditions behind the caudal part of the subgerminal space, at the end of the intrauterine development. Indeed, the nucleus of Pander (δ-ooplasm) and surrounding y-ooplasm, during the oblique positioning of the egg yolk ball, shift temporally or definitively in the future caudal direction [56,57].

Figures. 9A. 9B. 9C. 9D. 9E. 9F. The represented experiments indicate that neuro-gastrulation phenomena in the upper layer can been restored by subgerminal ooplasm in endophyll-free quadrants [24].
By placing germ discs (or fragments containing the deep central part of it) from unfertilized laid or extracted quail eggs on the deep side of the upper layer of isolated anti-sickle regions from unincubated chicken blastoderms, we observed in about 30% of the cases, the onset of the development of a miniature embryo formed, by chicken cells [85]. Our experimental results, suggest that the δ-ooplasm of the nucleus of Pander induces the overlying upper layer to form a pre-neural plate (Figure. 10A) [15].
More caudally, probably under the inductor influence of surrounding у-ooplasm, encircling the nucleus of Pander, other definitive chicken embryonic structures i.e. notochord, mesoblast, epiblast and neural plate develop (neuro-gastrulation phenomena) (Figure. 10B).

Figure 10A. Section through the cranial part of the embryo developed after 26 h of culture in vitro under the influence of a “thick” section of an unfertilized quail germ disc apposed on an isolated chicken anti-sickle region. NP: centre of the nucleus of Pander from the unfertilized quail germ disc. Note on top of the figure the thickened upper layer (preneural plate) and the endophyll layer (indicated by arrow heads) which is continuous with cells localized in the periphery of the nucleus of Pander, containing primordial yolk (PY) spheres; all the nuclei (both in the preneural plate as in the endophyll) are from chicken. Only above the endophyll, the upper layer shows a thickening with three rows of nuclei, whilst more peripherally the upper layer remains narrow (1 layer of nuclei). Feulgen staining.
Bar = 50 μm.

Figure 10B. Transverse section through a similar induced chicken embryo. A neural plate has developed in its cranial region: the chorda is indicated by an arrowhead; M: mesoblast; Y: yolk derived from unfertilized quail germ disc. Feulgen staining.
Bar = 50 μm.
Our experiments in birds seem to have some homology with the association experiments of isolated cortices from regions of unfertilized Xenopus eggs implanted into the ventro-equatorial core of recipient 8-cells Xenopus embryos [86].
1°. Culture of frontally hemi-sectioned unincubated chicken blastoderms (Figure. 11A): in the cranial half of such a blastoderm a centrally and caudally directed pre-neural plate developed, (80%), separated by a broad cavity from the underlying endophyll: no ingression phenomena were seen [87] (Figure. 11B).

Figure 11A. Schematic representation of the development of a frontally hemi-sectioned unincubated blastoderm in vitro. The caudal half blastoderm gives rise to a normal miniature embryo with neural plate (NP), primitive streak (PS), endophyllic crescent and coelomo-cardiovascular system (CCV): in the cranial half a preneural plate (PNP) develops from the upper layer (UL) under the influence of endophyll (E).

Figure 11B. Transverse section through the cranial half after one day of culture: the cranial half blastoderm, containing the upper layer (UL) of the anti-sickle region (AS) and some cranial underlying endophyll (E), usually gives rise to a preneural plate (with preneural groove) without ingression of the UL; at the rims of the blastoderm, two arrowheads indicate the contact zone between endophyll (E) and the upper layer (UL); PNG: preneural groove.
2°. Culture of isolated anti-sickle regions (mainly β-ooplasm containing) compared with the caudal isolated part of the blastoderm (Figure. 12): in the anti-sickle region, in 90% of the cases, only a strongly expanding epiblast and no other structures were seen; in the isolated caudal part of the blastoderm a normal embryo developed.

Figure 12. Schematic representation of the development in vitro of an isolated anti-sickle region (AS) and neighboring cranial area opaca (AO): usually only epiblast develops and no neurulation nor gastrulation take place.
In the caudal isolated part of the blastoderm, on the contrary, three ooplasms (β-, у- and δ-ooplasm) are fully present in undisturbed order and neuro-gastrulation phenomena occur with formation of a у-ooplasm-dependent-coelomo-cardiovascular system (CCV).
3°. Culture of quartered unincubated chicken blastoderms: comparison of a cranial, a caudal and a left and a right lateral quadrant (Figure. 13).

Figure 13. Schematic representation of the in vitro development of the four isolated quadrants of an unincubated chicken blastoderm. The caudal quadrant develops in a miniature embryo with area vasculosa caudalis (AVC), but usually no heart appears (absence of the sickle horns). In the lateral quadrants, usually hemi-embryos with hemi-PS (indicated by thick curved hemi-arrow in the cut edge) develop. In the cranial quadrant, usually, only appears a preneural plate and no ingression phenomena (gastrulation) are seen; NP: neural plate.
In the caudal isolated quadrant, a normal miniature embryo develops in every case since all the elementary tissues (UL, RS, endophyll and corresponding ooplasms) are present.
In the cranial isolated quadrant in 80% of the cases a pre-neural plate and underlying endophyll developed.
In the lateral quadrants, hemi-embryos developed with hemi-PS in the rim of the sectioned edge (as was described by Callebaut et al. [23,61].
Large ooplasmic structures (α-, β-, y-, δ-) visible in the oocytal germ disc several days before the fertilization, can been recognized after fertilization in the form of cell lineages in the early blastoderm and during further shaping and patterning of the embryo. The embryo proper (β-ooplasm containing) develops from the upper layer (somatic) cells of the area centralis. The equator-like disposed Wedge granules layer gives rise to the circularly localized y-ooplasm (interrupted by the presence and breakdown of the germinal vesicle). The densely packed y-ooplasm material is finally found in the Rauber’s sickle, the lateral and rostral marginal endoblast. The Rauber’s sickle material induces and mechanically shapes the upper layer cells in the area centralis mainly influencing the truncal region (body axis and segmentation of the embryo (Hox genes expression).
In the cranial region, the endophyll (primary hypoblast) has a signaling function inducing the head formation and its orientation. It induces also preneurulation phenomena [27,87]. So, the neural tube expresses Otx-2 in the future forebrain region [67]. It is mainly in the contact zones between the different ooplasms and/or subgerminal ooplasm (all or not by dislocation) that morphogenetic phenomena start. For instance, the contact zone between Rauber’s sickle material and overlying upper layer or between RS material and underlying subgerminal y-ooplasm. Also, interaction between the y-ooplasm-containing sickle endoblast (derived from Rauber’s sickle) and the more cranial endophyll (δ-ooplasm containing) has a starter role on the development of the primitive streak and the caudo-cephalic axis formation. Also mosaic versus regulation behavior in avian blastoderms depends on the spatial distribution of Rauber’s sickle material, relative to the presence or absence of neighboring upper layer cells [16-17]. Thus, apart from cellular migration phenomena also mechanical interactions between the neighboring different ooplasm-containing structures is obvious. At the unincubated blastoderm stage, clearly no complete possibility of regulation exists since after total or partial removal of some cell lineages no restoration of the involved structures takes place [18].
The authors thank Mr. David Malan for excellent photographic processing of the figures.
- Wetzel R (1924) Über den Primitive knoten des Hühnchens. Verh Phys Med Ges Würzburg 49: 227-233.
- Wetzel R (1929) Untersuchungen am Hühnchen. Die Entwicklung des Keims während der ersten beiden Bruttage. Wilhelm Roux Arch Entwickl Mech Org 119: 188-321. [Crossref]
- Waddington CH (1930) Developmental mechanics of chicken and duck embryos. Nature 125: 924-925.
- Waddington CH (1932) Experiments on the development of chick and duck embryos, cultivated in vitro. Philosophical Transactions. R Soc London 221: 179-230.
- Waddington CH, Schmidt GA (1933) Induction by heteroplastic grafts of the primitive streak in birds. Wilhelm Roux Arch Entwickl Mech Org 128: 522-563. [Crossref]
- Hara K (1961) Regional neural differentiation induced by prechordal and presumptive chordal mesoderm in the chick embryo. Proefschrift, Utrecht, Netherlands.
- Hara K (1978) Spemann’s Organizer in Birds. In: Nakamura O & Toivonen S (Eds.) “Organizer. A milestone of a half-century from Spemann”. Elsevier NH, Amsterdam, Netherlands.
- Eyal-Giladi H, Khaner O (1989) The chick’s marginal zone and primitive streak formation. Dev Biol 134: 215-221. [Crossref]
- Bachvarova RF, Skromne I, Stern CD (1998) Induction of primitive streak and Hensen's node by the posterior marginal zone in the early chick embryo. Development 125: 3521-3534. [Crossref]
- Callebaut M, Van Nueten E (1994) Rauber's (Koller's) sickle: the early gastrulation organizer of the avian blastoderm. Eur J Morphol 32: 35-48. [Crossref]
- Callebaut M, van Nueten E, Harrisson F, van Nassauw L, Schrevens A, et al. (1997) Avian gastrulation and neurulation are not impaired by the removal of the marginal zone at the unincubated blastoderm stage. Eur J Morphol 35: 69-77. [Crossref]
- Callebaut M, van Nueten E, Van Nassauw, L., Bortier, H. And Harrisson F (1998b) Only the endophyll-Rauber’s sickle complex and not cells derived from the caudal marginal zone induce a primitive streak in the upper layer of avian blastoderms. Reprod Nutr Development 38: 449-463.
- Callebaut M, van Nueten E, Bortier, H. And Harrisson F (2002c) Avian sickle endoblast induces gastrulation or neurulation in the isolated area centralis or isolated anti-sickle region respectively. Eur J Morphology 40: 1-13. [Crossref]
- Callebaut M (2005) Origin, fate and function of the components of the avian germ disc region and early blastoderm: role of ooplasmic determinants. Dev Dynam 233: 1194-1216. [Crossref]
- Callebaut M, van Nueten E, Harrisson F, Bortier H (2004) Induction and improved embryonic development by the nucleus of Pander in associated avian blastoderm parts: influence of d or ? ooplasm. J Morphology 260: 201-208.
- Callebaut M, van Nueten E, Harrisson F, Bortier H (2007) Mosaic versus regulation development in avian blastoderms depends on the spatial distribution of Rauber’s sickle material. J Morphology 268: 614-623. [Crossref]
- Callebaut M, van Nueten E, Harrisson F. Hubens G (2010a) Mosaic and regulation phenomena during the early formation of the chick blastoderm. Int J Zoology 10: 10.
- Callebaut M, van Nueten E, Hubens G, Harrisson F (2010b) Early formation of the coelomo-cardiovascular complex in the chick blastoderm. Belgian J Zoology 140: 65-73.
- Rauber A (1876) &Uu2021 Copyright OAT. All rights reservs im Entwicklungsplan. W Engelsman, Leipzig, Germany.
- Rauber A (1879) Formbildung und Formstörung in der Entwicklung von Wirbeltieren. Morph Jahr 5: 661-705.
- Koller C (1882) Untersuchungen über die Blätterbildung im Hühnerkeim. Archives Mikrosk. Anat 20: 171-211.
- Callebaut M, van Nueten E, Van Nassauw L, Hubens G (2013a) Earliest Anlagen of the area vasculosa and heart: Regional influences of “extraembryonic” (yolk rich) tissues. Trends Dev Biol 7: 1-13.
- Callebaut M (2015) From ooplasms to embryo. The “embryo in box” model. Edit Acco Leuven. Belgium.
- Callebaut M, van Nueten E, Bortier H, Harrisson F, van Nassauw L (1996) Map of the Anlage fields in the avian unincubated blastoderm. Eur J Morphol 34: 347-361. [Crossref]
- Izpisúa-Belmonte JC, De Robertis EM, Storey KG, Stern CD (1993) The homeobox gene goosecoid and the origin of organizer cells in the early chick blastoderm. Cell 74: 645-659. [Crossref]
- Nieuwkoop PD (1973) The organization center of the amphibian embryo: its origin, spatial organization, and morphogenetic action. Adv Morphog 10: 1-39. [Crossref]
- Callebaut M, van Nueten E, Harrisson F, van Nassauw L, Bortier H (1999) Endophyll orients and organizes the early head region of the avian embryo. Eur J Morphol 37: 37-52. [Crossref]
- Carlson B (2004) Human Embryology and Developmental Biology. 3rd (edn). Elsevier Mosby.
- Callebaut M (1974) La formation de l’oocyte d’oiseau. Etude autoradiographique chez la caille japonaise (Coturnix coturnix japonica) pondeuse à l’aide de la? H-leucine. Archives Biologie 85: 201-233.
- Callebaut M, Harrisson F, Vakaet L (1981) Peripheral avian yolk assemblage and its persistence in the blastoderm, studied by trypan blue-induced fluorescence. Anat Embryol (Berl) 163: 55-69. [Crossref]
- Harrisson F, Callebaut M, Vakaet L (1981) Microspectrographic analysis of Trypan-blue induced fluorescence in oocytes of the Japanese quail. Histochemistry 72: 563-578. [Crossref]
- Callebaut M (1983) The constituent oocytal layers of the avian germ and the origin of the primordial germ cell yolk. Arch Anat Microsc Morphol Exp 72: 199-214. [Crossref]
- Gilbert AB (1971) The ovary In: Bell DJ, Freeman BM (eds). Physiology and Biochemistry of the domestic fowl 3, 1st ed. Academic Press, New York, USA: 1163-1208.
- Pander C (1817) Historiam metamorphoseos quam ovum incubatum prioribus quinque diebus subit. Wirceburgi: FE Nitribitt :69.
- Von Baer K (1828) Über die Entwicklungsgeschichte der Thiere. Beobachtung und Reflexion Entwicklungsgeschichte des Hühnchens in Ei. Bornträger Verl. Königsberg.
- Tsunekawa N, Naito M, Sakai Y, Nishida T, Noce T (2000) Isolation of chicken vasa homolog gene and tracing the origin of primordial germ cells. Development 127: 2741-2750. [Crossref]
- Callebaut M (1983b) Electron microscope study of TICOS (H-thymidine incorporating cytoplasmic organelles) in the germinal disc of the post-lampbrush oocytes of Japanese quail. IRCS Medical Science 11: 491-492.
- Callebaut M (1984b) Avian primordial cells contain yolk from the nucleus of Pander. IRCS Medical Science 12: 730-731.
- Callebaut M (1987) Ooplasmic localization and segregation in quail germs: fate of the four ooplasms. Archives Biologie (Bruxelles) 98: 441-473.
- Callebaut M, Vakaet L (1981) Fluorescent yolk marking of the primary gonocytes in quail blastoderms by administration of Trypan-blue, during late oogenesis. IRCS Medical Science 12: 730-731.
- Callebaut M (1984a) Evolution of paranuclear sudanophilic organelles in quail oocytes. IRCS Medical Science 12: 1040-1041.
- Eyal-Giladi H, Ginsburg M, Farbarov A (1981) Avian primordial germ cells are of epiblastic origin. J Embryol Exp Morphol 65: 139-147. [Crossref]
- Curtis AS (1960) Cortical grafting in Xenopus laevis. J Embryol Exp Morphol 8: 163-173. [Crossref]
- Curtis AS (1965) Cortical inheritance in the amphibian Xenopus laevis: preliminary results. Arch Biol (Liege) 76: 523-546. [Crossref]
- Callebaut M (1973) Marquage intense de la couche des Wedge granules dans les oocytes en prématuration de la caille japonaise (Coturnix coturnix japonica) après injection de L-leucine-³H. Experientia 29: 846-847.
- Balbiani EG (1864) Sur la constitution du germe dans l’oeuf animal avant la fécondation. CR Hebdo Sciences Acad Sci 58: 584.
- Guraya SS (1962) The structure and function of the so-called yolk-nucleus in the oogenesis of birds. Quart J Micr Sci 103: 411-415.
- Guraya SS (1979) Recent advances in the morphology, cytochemistry, and function of Balbiani's vitelline body in animal oocytes. Int Rev Cytol 59: 249-321. [Crossref]
- Guraya SS (1989) Ovarian follicles in Reptiles and Birds. Zoophysiology 24. Springer Verlag, Berlin, Germany.
- de Smedt V, Szöllösi D, Kloc M (2000) The balbiani body: asymmetry in the mammalian oocyte. Genesis 26: 208-212. [Crossref]
- Kloc M, Spohr G, Etkin LD (1993) Translocation of repetitive RNA sequences with the germ plasm in Xenopus oocytes. Science 262: 1712-1714. [Crossref]
- Kloc M, Etkin LD (1995) Two distinct pathways for the localization of RNA sequences at the vegetal cortex in Xenopus oocytes. Development 121: 287-297. [Crossref]
- Callebaut M (1975) Bijdrage tot de studie van de oogenesis van de vogels.PhD Thesis, RUCA: 1-320.
- Bounoure L (1939) L’origine des cellules reproductrices et le problème de la lignée germinale. Collection des actualités, Paris, France.
- Eyal-Giladi H (1997) Establishment of the axis in chordates: facts and speculations. Development 124: 2285-2296. [Crossref]
- Callebaut ME (1993) Early eccentricity in gravitationally oriented quail germs. Eur J Morphol 31: 5-8. [Crossref]
- Callebaut M (1993b) Unequal caudocephalic ooplasmic uptake and eccentric formation of the subgerminal space below unincubated quail blastoderms presenting a Koller’s sickle. Belgian J Zoology 123: 107-112.
- Callebaut M (1994) Relationship between the avian blastoderm and the subgerminal ooplasm. Eur Arch Biology 105: 111-123.
- Callebaut M, van Nueten E, Bortier, H. And Harrisson F (2002a) In the absence of Rauber’s sickle material, no blood islands are formed in the avian blastoderm. J Morphology 253: 132-147.
- Callebaut M, van Nueten E, Harrisson F, Bortier H (2002b) Rauber’s sickle and not the caudal marginal zone induces a primitive streak, blood vessels, blood cell formation and coelomic vesicles in avian blastoderms. Eur J Morphology 48: 275-282.
- Callebaut M, van Nueten E, Van Nassauw L, Hubens G (2013b) The original predisposed “extraembryonic” distribution of the surrounding a-?-d-ooplasms regulates the patterning of the embryo proper (ß-ooplasm). Trends Dev Biol 7: 33-49.
- Callebaut M, van Nueten E, van Passel H, Harrisson F, Bortier H (2006) Early steps in neural development. J Morphol 267: 793-802. [Crossref]
- Callebaut M (2008) A Review. Historical evolution of preformistic versus neoformistic (epigenetic) thinking in embryology. Belgian J Zoology 138: 20-35.
- Callebaut M, Harrisson F, Bortier H (2001) Effect of gravity on the interaction between the avian germ and neighbouring ooplasm in inverted egg yolk balls. Eur J Morphol 39: 27-38. [Crossref]
- Harrisson F, Callebaut M, Vakaet L (1991) Features of Polyingression and primitive streak ingression through the basal lamina in the chicken blastoderm. Anat Rec 229: 369-383. [Crossref]
- Mogi K, Tayoizumi R, Takeuchi S (1998) Hypoblast cells of chick pre-streak embryos invade basement membrane analogous in vitro: implications for hypoblast layer formation. Dev Growth Differ 40: 2009-1019.
- Boettger T, Knoetgen H, Wittler L, Kessel M (2001) The avian organizer. Int J Dev Biol 45: 281-287. [Crossref]
- Roeser T, Stein S, Kessel M (1999) Nuclear localization of ß-catenin in normal and LiCI exposed chick embryos. Development 126: 2955-2965. [Crossref]
- Willier BH, Rawles M (1935) Organ-forming areas of the early chick blastoderm. Proc Soc Exp Biology 32: 1293-1296. [Crossref]
- Ebert JD (1953) An Analysis of the Synthesis and Distribution of the Contractile Protein, Myosin, in the Development of the Heart. Proc Natl Acad Sci USA 39: 333-344. [Crossref]
- Rosenquist GC (1966) A radioautograhic study of labeled grafts in the chick blastoderm. Contrib Embryol 38: 71-110.
- Dehaan R (1963) Migration patterns of the precardiac mesoderm in the early chick embrvo. Exp Cell Res 29: 544-560. [Crossref]
- De Haan RL (1965) Morphogenesis of the vertebrate heart. In Organogenesis. (Eds.) De Haan, H Ursprung Holt, Rinehart and Winston. New York, USA: 377-420.
- De Haan RL (1968) Emergence of form and function in the embryonic heart. Dev Biol Suppl 2: 208-250.
- Lopez-Sanchez C, Garcia-Martinez V, Schoenwolf GC (2001) Localization of cells of the prospective neural plate, heart and somites within the primitive streak and epiblast of avian embryos at intermediate primitive streak stages. Cells Tissues Organs 169: 334-346. [Crossref]
- Bellairs R (1971) Developmental Processes in Higher vertebrates.Logos Press Limited.
- Eichmann A, Corbel L, Pardanaud C, Breant D, Moyon D, et al. (2000) Hemangioblastic precursors in the avian embryo. Curr Top Microbiol Immunol 251: 83-90. [Crossref]
- New DAT (1955) A new technique for the cultivation of the chick embryo in vitro. J Embryol Exp Morphology 7: 146-164.
- Vakaet L (1962) Some new data concerning the formation of the definitive endoblast in the chick embryo. J Embryol Exp Morphol 10: 38-57. [Crossref]
- Vakaet L (1962b) Morfologische en experimentele Studie over de pregastrulatie en gastrulatie der Vogelkiem. Thesis, Arscia, Brussels.
- Callebaut M, van Nueten E (1995) Gastrulation inducing potencies of endophyll and Rauber’s sickle in isolated caudocranially oriented prestreak avian blastoderm quadrants (or fragments) in vitro. Eur J Morphology 33: 221-235.
- Callebaut M, van Nueten E, Bortier H, Harrisson F (2003) Competitive inhibition by Rauber’s sickle, primitive streak and/or (pre)neural plate inducing effects of sickle endoblast in avian blastoderms. J Morphology 257: 364-374. [Crossref]
- Callebaut M, van Nueten E, Bortier H, Harrisson F, van Nassauw L, et al. (1997a) Spatial relationship between endophyll, primordial germ cells, sickle endoblast and upper layer in cultured avian blastoderms. Reprod Nutr Development 37: 293-304. [Crossref]
- Sanders EJ, Bellairs R, Portch PA (1978) In vivo and in vitro studies on the hypoblast and definitive endoblast of avian embryos. J Embryol Exp Morphol 46: 187-205. [Crossref]
- Callebaut M, van Nueten E, Harrisson F, Bortier H (2000) Activation of avian embryo formation by unfertilized quail germ discs: comparison with early Amphibian development. Reprod Nutr Development 40: 597-606. [Crossref]
- Kageura H (1997) Activation of dorsal development by contact between dorsal determination and the equatorial core cytoplasm in eggs of Xenopus laevis. Development, 124: 1543-1551.
- Callebaut M, van Nueten E (1993) Differential influence of the endophyll and sickle of Koller material on the upper layer in frontally hemi-sectioned avian blastoderms in vitro. Eur Arch Biology 104: 63-68.





























