Novelty statement
- Navigating the myriad treatment options available to patients with relapsed/refractory AML can paint a confusing picture. This review provides a broad framework to understand the current management of remission-reinduction in relapsed/refractory AML (r/r AML).
- The numerous chemotherapeutic regimens available to patients in the r/r AML setting for remission-reinduction are elaborated, compared and contrasted, so that readers can better understand how informed treatment decisions are made by appreciating the nuances that guide these decisions. To this end, a focus on the mechanism of action of common agents employed and their toxicities is also discussed.
- Targeted therapies employed in specific AML populations are also reviewed similarly and help provide a framework to understand the options available to r/r AML patients and those specific populations that are most likely to benefit from them
- A brief overview is also provided regarding select immunotherapeutic options that are also being actively investigated in the management of r/r AML
Abstract
Introduction: Acute myelogenous leukemia [AML] is a heterogenous group of primary hematopoietic neoplasms arising from myeloid precursor cells. Up to 50% of patients failed to achieve remission with initial therapy and go on to develop relapsed/refractory AML [R/R AML]. Current standard of care remains enrollment in a clinical trial in view of the paucity of evidence surrounding it clearly superior treatment modality, and the therapy which provides the best chance for cure is allogeneic hematopoietic stem cell transplantation [HSCT], with much of everyday clinical decision-making in R/R AML surrounding the choice of the least toxic regimen that could achieve remission-reinduction and enable prompt HSCT.
Discussion: We discuss in this contemporary review a wide variety of treatment modalities currently employed in order to achieve this goal. Traditional cytotoxic chemotherapeutic agents such as cytarabine, fludarabine and daunorubicin are reviewed amongst several others, with a focus on commonly employed treatment regimens in the salvage setting such as HiDAC, FLA/FLAG, MEC and GCLAC, to name a few. We then turn our attention to newer targeted therapies that have shown promise in specific patient populations such as the IDH inhibitors ivosidenib and enasidenib, and FLT3 inhibitors such as gilteritinib and quizartinib. Several other targeted agents that have been studied in the R/R AML setting are also discussed, with some of these agents being primarily used in entirely different diseases altogether. Lastly we turned our attention to a few immunotherapeutic agents employed in the R/R in the setting, the CD33 inhibitors and the novel bispecific antibodies.
Conclusion: The difficulties of treating R/R AML are compounded by the fact that many patients are poor candidates in view of prohibitive comorbidities and poor performance status. It appears increasingly clear that approaching AML as a homogenous disease entity is unsatisfactory in view of the variations in such disease factors as cytogenetic and molecular markers, age, and disease severity at presentation; all of which contribute significantly to heterogeneity of the disease. The future direction of tackling AML would likely require tailored therapy following advances in technology such as molecular profiling, drug sensitivity and resistance testing.
Keywords
acute myeloid leukemia, relapsed/refractory AML, salvage therapy AML, malignant hematology, chemotherapy regimens AML, immunotherapy AML, targeted therapy AML
Introduction
Acute myeloid leukemia (AML) is a heterogeneous group of primary hematopoietic neoplasms arising primarily from cells committed to the myeloid line of cellular development. Although the response to treatment and overall prognosis is variable, dependent on several patient and tumor specific factors as age, performance status and karyotype, 30 – 50% of patients with newly diagnosed AML will fail to attain a complete remission (CR) with intensive chemotherapy. Additionally, up to 50% of patients who initially achieve a CR subsequently develop relapsed or refractory AML (r/r AML), notably in elderly populations. These patients generally have a poor prognosis.
The first line of treatment and current standard of care for these patients remains enrollment in a clinical trial. In new onset AML, as in r/r AML, the therapy which provides the best chance for cure is an allogenic hematopoietic stem cell transplantation [HCT]. As such remission reinduction involves the selection of the most active and least toxic regimen that can achieve disease control and enable prompt HCT. Although remission reinduction may not be required in all patients, especially those who receive myeloablative preparations prior to HSCT, all patients who receive nonmyeloablative conditioning regimens will require remission-reinduction prior to HSCT, ideally having achieved a CR. A graphical representation of this management process is provided (Figure 1).
A wide variety of different treatment regimens have been studied to improve outcomes in patients with r/r AML, although there appears to be no single superior approach. We will attempt to review traditional chemotherapeutic agents used in the non-APL AML salvage setting, as well as targeted single agents and immunotherapeutic agents, amongst others, employed in contemporary practice. A graphical summary of these treatment options is provided (Figure 2).
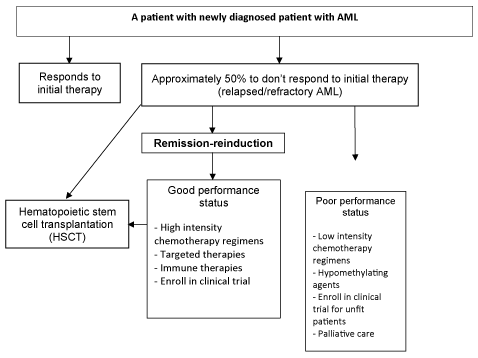
Figure 1: A framework for the management of remission-reinduction in relapsed/refractory acute myeloid leukemia
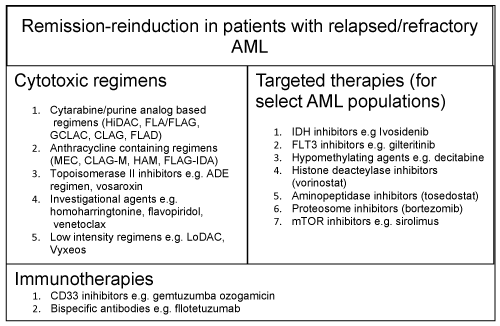
Figure 2: A summary of the modalities discussed that are employed in remission-reinduction
Cytotoxic therapeutic agents
Cytarabine
Cytarabine, or Ara-C, cytosine arabinoside, is a chemotherapeutic agent that primarily inhibits DNA synthesis. Cytarabine, being a pyrimidine analog gain entry into cells and is converted to its active compound, azacitidine triphosphate, following which it is incorporated into beginning. If primary action is inhibition of DNA polymerase resulting in decreased synthesis and repair of DNA, and the specific to the S phase of the cell cycle. Its metabolism is primarily hepatic with urinary excretion of active metabolites, with a half-life of 20 minutes. Notable concerns related to administration of the medication include myelosuppression, GI toxicities and cytarabine syndrome characterized by fever, myalgia, maculopapular rash and generally occurs 6 to 12 hours following administration.
Cytarabine is very commonly used either alone or in combination with other chemotherapeutic agents, notably anthracyclines, both for remission induction in newly diagnosed AML as well as salvage therapy in r/r AML. The HiDAC regimen or high dose cytarabine given has been shown to achieve CR and up to 35 to 40% of patients resistant to conventional dose cytarabine regimens. In general, patients may receive HiDAC in the salvage setting if they have not yet received it either with or without an anthracycline. However, in patients who suffer a late relapse more than 18 months from initial CR, although they may achieve a second CR by retreatment with HiDAC if initially treated with the same, reported CR rates are 32 to 47% [1]. Interestingly, the combination of HiDAC plus an anthracycline may produce higher response rates with side effects appearing to be similar in both regimens, although toxicity is prohibitively high in patients over the age of 60 [2]. Unlike HiDAC, reinduction with cytarabine plus anthracycline may be employed for those who receive it initially and undergo CR that persists for more than 1 year. CRs in this setting have been reported at approximately 50% [3].
Cytarabine serves as the backbone for several salvage regimens including FLA/FLAG (Fludarabine. Cytarabine/G-CSF), CLAG/M (Cladribine, Cytarabine, G-CSF/mitoxantrone), GCLAC (G-CSF, clofarabine, cytarabine), FLAD (Fludarabine, cytarabine, liposomal daunorubicin), and FLAM (Flavopiridol, cytarabine, mitoxantrone) to name some of the more commonly employed. A newer agent, elacytarabine, which is an acid ester of cytarabine, has not been found to improve CR or OS rates compared to standard regimens [4].
Purine analogs: Fludarabine, clofarabine and cladribine
Fludarabine, clofarabine and cladribine are commonly employed purine analogs in several salvage regimens in the treatment of r/r AML. Fludarabine inhibits DNA synthesis through inhibition of DNA polymerase and ribonucleotide reductase, as well as inhibition of DNA primase and DNA ligase I. Clofarabine, in addition to ribonucleotide reductase inhibition, inhibits the DNA repair process and alters mitochondrial membrane permeability releasing inducing factor and cytochrome C. Cladribine is cell cycle nonspecific, and its incorporation into DNA results in strand breakage and shutdown of DNA synthesis and repair.
In addition to myelosuppression, fludarabine may be neurotoxic. Autoimmune phenomena including hemolytic anemia and Evans syndrome have also been described [5]. Renal dose adjustment may be necessary, common to all the purine analogues. Fludarabine is most commonly employed as part of the FLA/FLAG regimens. Idarubicin may also be combined in the widely used FLAG–IDA regimen, with reported CR rates of around 50% [6]. FLAG-IDA is a frequently used control arm in clinical trials for r/r AML. In atleast one study no significant difference in CR rates were observed between FLAG and FLAG-IDA [7].
Fludarabine has been shown to augment the rate of synthesis of ara-CTP in blast cells when administered prior to cytarabine [8]. Despite addition of G-CSF to FLA, reported CR rates did not differ significantly, being 61% and 58% respectively [9]. The FLAD regimen (Fludarabine, cytarabine and liposomal daunorubicin) achieved an overall CR rate of 53% in one study, and as 58% of these patients went on to undergo allogeneic HSCT, this regimen may have utility as a bridge to transplant [10].
Clofarabine administration has been linked to a cytokine release syndrome with subsequent capillary leak and organ dysfunction, which may prove fatal. Additionally, tumor lysis syndrome is an important consideration with this agent. Clofarabine may be administered with intermediate dose cytarabine, with CR rates of 35% to 51% being reported, as evidenced by at least one study [11].
Cladribine is employed in a curious array of pathologies besides refractory AML, including refractory T-cell large granular lymphocytic leukemia and relapsing multiple sclerosis. Like fludarabine, it is myelotoxic and nephrotoxic. Regimens employing Cladribine include CLAM (Cladribine, cytarabine, mitoxantrone) and CLAG (Cladribine, Cytarabine, G-CSF), with reported CRs of 40 to 50% [12]. Even higher CRs of up to 58% were reported with the CLAG-M (Cladribine, Cytarabine, G-CSF, mitoxantrone) regimen [13].
Anthracyclines and analogs: Daunorubicin, idarubicin and mitoxantrone
The anthracycline drugs daunorubicin and idarubicin inhibit DNA and RNA synthesis by intercalation between DNA base pairs and steric hindrance of local uncoiling of the double helix. Mitoxantrone is a structurally related anthracenedione that functions similarly to cause DNA strand crosslinkage, strand breaks and nucleic acid synthesis inhibition through steric hindrance.
Mitoxantrone has found favor over the anthracyclines as it has a reduced incidence of cardiotoxicity and gastrointestinal side effects [14], and as such has replaced daunorubicin in combinations with cytarabine and etoposide. Regardless, all patients on anthracyclines/mitoxantrone require comprehensive cardiovascular assessment and monitoring. Hepatotoxicity is also a concern with these medications. Mitoxantrone with etoposide is commonly used for salvage therapy, with CR rates of approximately 40% being reported [15]. The MEC regimen (mitoxantrone, etoposide, cytarabine) has been extensively evaluated, with CR rates of upto 66% being reported [16]. Mitoxantrone has also been extensively used in combination with high and intermediate dose cytarabine (HAM regimen), and notably patients over 60 years achieved a CR rate of 76% with this regimen [17].
Liposomal formulations of daunorubicin have the advantage that higher effective plasma concentrations of the drug can be delivered with subsequent increased concentration in the bone marrow. Aside from the FLAD regimen, CPX-351 is a liposomal formation of cytarabine and daunorubicin in a 5:1 molar ratio. Although no statistical difference in 1-year survival between CPX-351 and standard salvage chemotherapies was found, CPX-351 excited attention as it was found to increase CR rates in subgroups with poor risk features including elderly age and adverse cytogenetics compared to standard chemotherapy, 47.7% and 33.3% respectively [18], although this study did not evaluate the drug in the r/r AML setting.
Idarubicin as a component of the FLAG-IDA regimen was discussed under the purine analogs. The addition of idarubicin may be important as FLA alone was inferior to cytarabine, daunorubicin and etoposide reinduction [19].
Topoisomerase II inhibitors: Etoposide and vosaroxin
Etoposide is a type II topoisomerase inhibitor and appears to cause DNA strand breakage, with subsequent cell cycle arrest in the S phase. As a chemotherapeutic agent, it has found wide application in both solid and liquid malignancies, notably small cell lung and testicular, although it is almost universally given in combination with other agents for r/r AML. Renal dose adjustment is necessary as it is nephrotoxic. Like most chemotherapeutic agents, it causes myelosuppression and GI side effects.
Aside from the MEC regimen, high dose etoposide has been evaluated to have demonstrable efficacy in conjunction with cyclophosphamide, with a reported CR rate of up to 42% [20], with mucosal toxicity being dose limiting. The ADE regimen (Cytarabine, daunorubicin, etoposide) may be administered together as part of a standard regimen or sequentially. The standard administration has been shown to have improved CR rates (54% vs 34%) with improved 3-year survival (12% vs 6%), and may therefore be superior [21].
Vosaroxin is a novel quinolone derivative that inhibits DNA topoisomerase II, and aside from being investigated as a therapeutic target for primary AML [22] has been found to improve CR rates in combination with cytarabine in r/r AML, as compared to cytarabine with placebo (30% vs 16% respectively), although treatment related adverse events and deaths were also higher in the treatment groups [23].
Other cytotoxic agents: Homoharringtonine, flavopiridol and venetoclax
Omacetaxine mepesuccinate, previously known as homoharringtonine, is a plant alkaloid derived from Cephalotaxus fortune. It is a protein translation inhibitor, and inhibits correct positioning of amino acids during the initial step of protein translation [24]. Although it has been approved for TKI resistant CML, its role in r/r AML is still a subject of active investigation. The HAA regimen (Homoharringtonine, cytarabine, aclarubicin) was evaluated in one study with a CR rate of 76% [25].
Flavopiridol, also known as alvocidib, is a synthetic analog of a naturally occurring flavonoid alkaloid isolated from Dysoxylum binectariferum tree bark. It has properties as a cyclin dependent kinase inhibitor and leads to inhibition of positive transcription elongation factor b [26]. The FLAM regimen (Flavopiridol, cytarabine and mitoxantrone) has demonstrated particularly efficacy as compared to carboplatin-topotecan and sirolimus-MEC per a phase II study, with a reported CR rate of 28% vs 14% and 15% [27].
Venetoclax, an oral formulation, is a B cell leukemia/lymphoma-2 (BCL-2) inhibitor. BCL-2 display anti-apoptotic activity and promotes myeloblast survival; hence its inhibition presents an attractive target to combat AML. Although venetoclax has shown impressive results in the treatment of CLL, monotherapy for r/r AML is underwhelming, with an overall CR rate of 19% [28]. Combination therapy for r/r AML is also unimpressive, although venetoclax with HMAs, in particular azacitidine has shown higher CR rates than with decitabine or low dose cytarabine (17.9% vs 6.7% and 0% respectively) [29].
A table comparing the chemotherapeutic regimens discussed so far has been provided for ease of reference in table 1.
Table 1: A relative comparison of the different chemotherapeutic regimens discussed in the management of relapsed/refractory acute myeloid leukemia.
Regimen |
Study reference |
Study design |
Number of relapsed/refractory AML patients |
Reported CR rates (%) |
HiDAC |
[4] |
Phase II |
78 |
63 |
Cytarabine + anthracycline |
[6] |
Phase III |
667 |
50 |
Elacytarabine |
[24] |
Phase III |
381 |
23 |
FLAG-IDA |
[22] |
Phase II |
46 |
52 |
FLA |
[9] |
Phase III |
405 |
61 |
FLAD |
[11] |
Phase II |
41 |
53 |
Cytarabine + clofarabine |
[12] |
Phase II |
21 |
43 |
CLAG/CLAM |
[13] |
Phase II |
24 |
44 |
CLAG-M |
[14] |
Phase II |
118 |
58 |
Mitoxantrone + etoposide |
[18] |
Phase II |
61 |
43 |
MEC |
[17] |
Phase II |
32 |
66 |
HAM |
[19] |
Phase II |
44 |
36 |
Etoposide + cyclophosphamide |
[21] |
Phase II |
40 |
42 |
ADE |
[23] |
Phase III |
235 |
43 |
Vosaroxin + cytarabine |
[29] |
Phase III |
711 |
30 |
HAA |
[26] |
Phase II |
46 |
80 |
FLAM |
[62] |
Phase II |
92 |
28 |
Venetoclax |
[30] |
Phase II |
32 |
6 |
HiDAC: High Dose Cytarabine; FLAG-IDA: Fludarabine, Cytarabine, G-CSF and Idarubicin; FLA: Fludarabine and cytarabine; FLAD: Fludarabine, Cytarabine and Liposomal Daunorubicin; CLAG: Clofarabine, Cytarabine and G-CSF; CLAM: Clofarabine, Cytarabine and Mitoxantrone; CLAG-M: Clofarabine, Cytarabine, G-CSF and Mitoxantrone; MEC: Mitoxantrone, Etoposide and Cytarabine; HAM: High and intermediate dose Cytarabine And Mitoxantrone; ADE: Cytarabine, Daunorubicin and Etoposide; HAA: Homoharrngtonine, Cytarabine and Aclarubicin; FLAM: Flavopiridol, Cytarabine and Mitoxantrone
Targeted therapies
Isocitrate dehydrogenase inhibitors (IDH1, IDH2): Ivosidenib, Enasidenib
Ivosidenib is an oral small molecule inhibitor of the mutant isocentric dehydrogenase 1 [IDH1] enzyme. IDH1 mutations in AML may have a frequency of up to 33% [30], and they lead to increased levels of 2-hydroxyglutarate and cells, which inhibits alpha-ketoglutarate-dependent enzymes that subsequently results in impaired hematopoietic differentiation. Consequently, ivosidenib in IDH1 mutated AML blood samples decreased intracellular levels of 2-hydroxybutyrate with reduced blast counts and induced myeloid differentiation with increased percentages of mature myeloid cells. Differentiation syndrome can occur in these patients and may be fatal, and QT prolongation is also a concern [31].
Enasidenib similarly is a small molecule inhibitor of IDH2 with subsequent reduction in blast count and increased maturation of myeloid cells, and has been noted to work independent of the mutational load of IDH2. Resistance to enasidenib may emerge through development of second site IDH 2 mutations which inhibit drug binding [32].
In patients with IDH1 mutated R/R AML, monotherapy with ivosidenib yielded CR rates of 21.8% in a phase 1 dose escalation and expansion trial, with an overall response rate of 41.6% [31]. Notably, transfusional independence was attained in 35% of patients who had achieved a response. Similarly, enasidenib as monotherapy for IDH2 mutant R/R AML was shown to achieve an overall response rate of 40.3% with a CR rate of 20.2% in a phase 1/2 dose escalation and expansion trial. 19.3% of those who attained CR had an overall survival of 19.7 months [33].
FMS-like tyrosine kinase receptor 3 inhibitors: Gilterinib, quizartinib
The FLT3 gene encodes a tyrosine kinase receptor that plays a key role in controlling proliferation and differentiation of hematopoietic cells. FLT3–ITD and FLT3–TKD oncogene mutations are common in AML with frequencies of up to 25% and 10% respectively [34]. First generation FLT3 inhibitors midostaurin and sorafenib function relatively non-selectively against FLT3, and although midostaurin has been approved as first-line therapy, both have little activity as monotherapy in relapsed patients. In contrast, next generation TKI's which include gilteritinib and quizartinib have shown antileukemic single agent activity [35], and gilteritinib is also FDA approved for r/r FLT3 mutated AML. Inhibition of FLT3 receptor signaling by gilteritinib induces apoptosis in these leukemic cells. As with IDH inhibitors, fatal differentiation syndrome and QTc prolongation are concerns with administration. Rare reports of posterior reversible encephalopathy syndrome and pancreatitis have been observed but not conclusively associated.
The ADMIRAL trial compared outcomes in patients with r/r FLT3 mutated AML who were administered either gilteritinib or standard salvage chemotherapy, with gilteritinib administration resulting in significantly longer survival than chemotherapy [9.3 versus 5.6 months], as well as a high percentage of patients with complete remission compared to salvage chemotherapy [21.1% versus 10.5%] [36]. Serious adverse effects were also reported less frequently in the gilteritinib group, the most common of which were cytopenias. Although quizartinib is not FDA approved for r/r FLT3 mutated AML, the QuANTUM-R trial comparing quizartinib and salvage chemotherapy showed a median overall survival benefit with quizartinib of 6.2 months versus 4.7 months with chemotherapy [37]. A phase 1 dose escalation and expansion study for r/r FLT3–ITD mutated AML elicited a complete remission rate of 37.5% in study participants [38].
Hypomethylating agents: azacitidine, decitabine
Decitabine and azacitidine are pyrimidine nucleoside analogs of cytidine that are incorporated into DNA and RNA and subsequently inhibit DNA/RNA methyltransferases. Reduced DNA/RNA methylation alters DNA gene expression including expression of tumor suppressor genes and decreases RNA stability and protein synthesis. Unlike many of the agents hitherto described, hypomethylating agents are by and large reserved as treatment options for patient's considered to be poor candidates for aggressive salvage therapy, such as those with poor performance status or significant comorbidities. As with traditional cytotoxics, bone marrow suppression is a concern. In contrast to decitabine, azacitidine may be nephrotoxic and hepatotoxic, requiring dosage adjustments in those with pre-existing impairments.
An outcomes review of r/r AML patients treated with azacitidine at three different French institutions showed a CR rate of 21%, with a bone marrow blast percentage less than 20% being identified as the only independent prognostic factor irrespective of age or performance status [39]. A study of decitabine administration in a cohort of 102 patients with r/r AML showed a CR rate of 15.7% with a median overall survival of 177 days [40].
Histone deacetylase inhibitors: Vorinostat
The histone deactylase enzymes HDAC1, HDAC2, HDAC3 and HDAC6 catalyze removal of acetyl groups from histones and transcription factors, and inhibition of the same results in altered chromatin structure and transcription factor activation with subsequent cell growth termination and apoptosis.
Vorinostat is a histone deactylase inhibitor approved for cutaneous T-cell lymphoma which, as monotherapy in RR–AML, was found to have a CR rate of only 4.5% in a group of 22 patients [41]. It fared better in a trial of combination therapy with cytarabine and etoposide with a CR rate of 46% [42].
Aminopepetidase inhibitors: Tosedostat
Aminopeptidases are endogenous metallo-enzymes that operate downstream of the ubiquitin-proteosome pathway that are responsible for the final step of intracellular protein degradation. Tosedostat, an M1 aminopeptidase enzyme family inhibitor, functions in a tumor selective manner and causes depletion of cellular amino acid pools, with resultant apoptosis.
Tosedostat monotherapy in the r/r AML setting has been evaluated with CR rates of 10% in a phase II study [43]. A trend for a higher response rate and survival was observed in patients with prior MDS or who had received hypomethylating agents for previous first induction, as well as patients who had multilineage AML compared to other AML types. Adverse events resulting in death were noted to be acute hepatitis, respiratory failure, pneumonia, atrial fibrillation and left ventricular dysfunction.
Proteosome inhibitors: Bortezomib
Bortezomib inhibits chymotrypsin-like activity at the 26S proteasome, with subsequent activation of signaling cascades that result in apoptosis of the cell. It is a drug commonly employed in multiple myeloma, with indications for cutaneous T cell lymphoma and mantle cell lymphoma as well. Aside from myelosuppression and peripheral neuropathy, new onset heart left ventricular dysfunction has also been reported with administration.
Although the role of bortezomib as monotherapy in r/r AML has yet to be elucidated, an expanded phase I trial of bortezomib + MEC (mitoxantrone, etoposide, cytarabine) in r/r AML patients demonstrated CR/CRi (complete remission with incomplete count recovery) rates of 52% [44]. Interestingly, of the five patients with RUNX1 mutations (a mutation associated with unfavorable outcomes and shorter survival times in MDS patients [45]), three (60%) achieved CR/ CRi, suggesting that bortezomib may have possible benefit in this difficult subset of pts
Mammalian target of rapamycin (mTOR) inhibitors: Sirolimus, everolimus
mTOR is a serine/threonine kinase involved in the regulation of cell growth and proliferation. mTOR inhibitors such as sirolimus (rapamycin) have found common use as immunosuppressive agents following organ transplantation. Although activating mutations or overexpression of mTOR has not been demonstrated in AML, studies have shown that high levels of PI3K products which activate Akt and mTOR, as well as phosphorylation of mTOR downstream targets, are constitutively activated in AML [46]. mTOR inhibitors sirolimus and everolimus bind to Fk-binding protein 12 (FKBP12) to form a complex that inhibits activation of mTOR activity.
Sirolimus monotherapy in one study was shown to have only a partial response rate of 44% in patients with r/r AML and poor risk AML [47]. A phase Ib/II study of everolimus in combination with azacitidine in r/r AML patients showed a CR/CRi rate of 12.5%, with dose limiting toxicity being observed in only 2 of the 40 patients in the study population [48]. In contrast, a phase Ib study of everolimus with conventional 7+3 daunorubicin and cytarabine induction chemotherapy in patients with first relapse AML demonstrated a CR rate of 68%, with <10% toxicity reported mainly involving the GI tract and lungs.
Immunotherapies
CD33 inhibitors: Gemtuzumab Ozogamicin (GO), lintuzumab
CD33 is expressed in over 80% of patients with AML [49], and as such is a promising target for immunotherapy. Gemtuzumab ozogamicin (GO) is a humanized CD-33 directed monoclonal antibody-drug conjugate, which is composed of the IgG4 kappa antibody gemtuzumab linked to a cytotoxic antibiotic calicheamicin derivative. GO binds to CD33 antigen which results in internalization of the antibody-antigen complex and subsequent release of the calicheamicin derivative. This derivative binds to DNA causing double-stranded breaks, inducing cell cycle arrest and apoptosis. Potentially fatal hepatic sinusoidal obstruction syndrome has been reported with use of GO.
Although the drug was initially withdrawn over safety concerns with full dosing, a phase II study of patients with CD33 positive first relapse AML who were administered fractional doses of GO demonstrated an excellent safety profile with a CR rate of 26% [50]. Of note, remission rates correlated with P-glycoprotein and MRP1 activity. Fractionated GO with standard dose cytarabine achieved even more impressive response rates in one study of late first relapse CD33 positive AML, with CR and CR with incomplete platelet recovery rates of 75% [51].
Bispecific antibodies: Flotetuzumab, AMG 330
Bispecific antibodies, a newer approach to immunotherapy, are antibodies which contain two antigen recognition sites that can be specific to two different antigens or two different epitopes of the same antigen. Bi-specific T-cell engager (BiTE) antibodies are bispecific antibodies constructed by linking the scFv fragments of two different monoclonal antibodies with a short flexible peptide linker, thereby allowing simultaneous binding of T–cell surface molecules and tumor cell antigens to promote tumor lysis. AMG 330 is one such BiTE directed against CD3+ T cells and CD33+ AML tumor cells with resultant T-cell activation and cytotoxicity.
A phase I dose escalation study of AMG 330 r/r AML patients proved to be well tolerated. Although cytokine release syndrome proved to be the most frequent adverse event (67%), it was reversible and occurred in a dose-dependent manner. 17% of patients developed CR or CRi, with 57% of these having an adverse cytogenetic risk profile [52].
Dual affinity retargeting proteins (DARTs) are bispecific antibodies formed by the heterodimerization of two Fv fragments (Fv1 contains the heavy chain region of antibody 1 and light chain region of antibody 2, whilst Fv2 contains the heavy chain region of antibody 2 and the light chain region of antibody 1). Similar to BiTE antibodies, they are bispecific, but have been shown to be more potent and stable that BiTEs [53]. Flotetuzumab is an investigational DART that engages CD3 on T-cells and C123, an IL3-α receptor subunit expressed in 60-80% of AML patients.
A phase II study of flotetuzumab in patients with r/r AML showed a combined CR/CRh (complete remission with partial hematologic recovery) rate of 26.7%, with median overall survival of 10.2 months. The most frequent reported adverse events were infusion related reactions and CRS that was largely mitigated with dexamethasone and tocilizumab pretreatment [54]. Of note, bone marrow transcriptomic analysis revealed a 10-gene signature that predicted response to flotetuzumab in this subset.
Discussion and closing remarks
AML remains a disease entity with a poor clinical outcome, and most patients diagnosed with AML will die because of it. Even those patients treated with curative intent induction chemotherapy and having achieved complete remission per currently defined endpoints only had a median overall survival of 20 months in one review of over 4000 patients with AML [55]. Compounding this difficulty is the fact that many patients are not candidates in view of poor performance status and prohibitive comorbidities, and these patients have limited treatment options, such as best supportive care and low intensity therapy with palliative intent.
Allogeneic hematopoietic stem cell transplantation remains the treatment strategy with the best chance of providing a cure, even though 5-year survival rates in such patients are reported at only 25% [56]. As such, HLA typing should be done as soon as R/R AML is identified, and a donor search initiated for HSCT to aid with the decision-making process.
The choice of conditioning regimen prior to HSCT varies significantly between institutions and according to experience. Myeloablative regimens with single agents or a combination of these, such as busulfan, melphalan and cyclophosphamide are often employed. Total body irradiation can also be used to achieve this goal in combination with these agents, with the goal being long-lasting and irreversible pancytopenia in preparation for HSCT. Patients may also not require a remission-reinduction regimen with myeloablative conditioning, as opposed to nonmyeloablative reduced intensity regimens. Eligibility criteria for HSCT vary significantly but patients with poor overall performance status, or moderate to severe organ impairment are usually considered ineligible. Achievement of a CR prior to HSCT is associated with the best survival outcomes and is generally preferred, to which end we credit the multiplicity of the treatment options hitherto discussed.
It is possible that R/R AML patients are by and large manifesting a refractory clone that has persisted despite successful initial therapy, and in view of this, it should not be assumed that the initial treatment was successful, but rather that it was not as effective as could be hoped. It seems increasingly evident that the concept of ‘remission’ in these patients is predicated on outmoded criteria, as they do not provide a sensitive enough assessment of AML disease burden [57]. Newer modalities such as minimal residual disease assays have helped to highlight this discrepancy, and also serve to explain the fact that despite the apparent success of many patients achieving a complete remission after induction therapy, their median overall survival remains less than two years [55].
Ultimately, there is no current standard of care in the management of AML, and the best treatment option in R/R AML in patients fit for intensive therapy would be enrollment in a clinical trial. Although AML has often been approached as a homogenous disease entity, variations in such disease factors as cytogenetic and molecular markers, age at presentation and disease severity on presentation all contribute to significant heterogeneity in this disease. It is increasingly being understood that tackling AML would require individualized therapy, and advances in technologies such as molecular profiling and drug sensitivity and resistance testing would perhaps be increasingly employed to tailor such therapy.
Author’s contributions
The authors meet the ICMJE authorship criteria. Prabasha Weeraddana and Meagan Josephs assisted with collecting the data. Vincent Rella and Kamila Bakirhan helped revise and edit the preliminary draft. Mohamed Zakee Mohamed Jiffry wrote the manuscript, supervised and approved the final version of the work to be published, and agrees to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
References
- Herzig RH, Lazarus HM, Wolff SN, Phillips GL, Herzig GP (1985) High-dose cytosine arabinoside therapy with and without anthracycline antibiotics for remission reinduction of acute nonlymphoblastic leukemia. J Clin Oncol 3: 992-997. [Crossref]
- Karanes C, Kopecky KJ, Head DR, Grever MR, Hynes HE, et al. (1999) A phase III comparison of high dose ARA-C (HIDAC) versus HIDAC plus mitoxantrone in the treatment of first relapsed or refractory acute myeloid leukemia Southwest Oncology Group Study. Leuk Res 23: 787-794. [Crossref]
- Breems DA, Van Putten WL, Huijgens PC, Ossenkoppele GJ, Verhoef GE, et al. (2005) Prognostic index for adult patients with acute myeloid leukemia in first relapse. J Clin Oncol 23: 1969-1978. [Crossref]
- Roboz GJ, Rosenblat T, Arellano M, Gobbi M, Altman JK, et al. (2014) International randomized phase III study of elacytarabine versus investigator choice in patients with relapsed/refractory acute myeloid leukemia. J Clin Oncol 32: 1919-1926. [Crossref]
- Sen K, Kalaycio M (1999) Evan's syndrome precipitated by fludarabine therapy in a case of CLL. Am J Hematol 61: 219. [Crossref]
- Pastore D, Specchia G, Carluccio P, Liso A, Mestice A, et al. (2003) FLAG-IDA in the treatment of refractory/relapsed acute myeloid leukemia: single-center experience. Ann Hematol 82: 231-235. [Crossref]
- Virchis A, Koh M, Rankin P, Mehta A, Potter M, et al. (2004) Fludarabine, cytosine arabinoside, granulocyte-colony stimulating factor with or without idarubicin in the treatment of high risk acute leukaemia or myelodysplastic syndromes. Br J Haematol 124: 26-32. [Crossref]
- Gandhi V, Estey E, Keating MJ, Plunkett W (1993) Fludarabine potentiates metabolism of cytarabine in patients with acute myelogenous leukemia during therapy. J Clin Oncol 11: 116-124. [Crossref]
- Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK (2006) Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107: 4614-4622. [Crossref]
- De Astis E, Clavio M, Raiola AM, Ghiso A, Guolo F, et al. (2014) Liposomal daunorubicin, fludarabine, and cytarabine (FLAD) as bridge therapy to stem cell transplant in relapsed and refractory acute leukemia. Ann Hematol 93: 2011-2018. [Crossref]
- Faderl S, Gandhi V, O'Brien S, Bonate P, Cortes J, et al. (2005) Results of a phase 1-2 study of clofarabine in combination with cytarabine (ara-C) in relapsed and refractory acute leukemias. Blood 105: 940-947. [Crossref]
- Martin MG, Welch JS, Augustin K, Hladnik L, DiPersio JF, et al. (2009) Cladribine in the treatment of acute myeloid leukemia: a single-institution experience. Clin Lymphoma Myeloma 9: 298-301. [Crossref]
- Wierzbowska A, Robak T, Pluta A, Wawrzyniak E, Cebula B, et al. (2008) Cladribine combined with high doses of arabinoside cytosine, mitoxantrone, and G-CSF (CLAG-M) is a highly effective salvage regimen in patients with refractory and relapsed acute myeloid leukemia of the poor risk: a final report of the Polish Adult Leukemia Group. Eur J Haematol 80: 115-126. [Crossref]
- Coufal N, Farnaes L (2011). Anthracyclines and Anthracenediones. In: Minev B. (eds) Cancer Management in Man: Chemotherapy, Biological Therapy, Hyperthermia and Supporting Measures. Cancer Growth and Progression, vol 13. Springer, Dordrecht. [https://doi.org/10.1007/978-90-481-9704-0_5]
- Ho AD, Lipp T, Ehninger G, Illiger HJ, Meyer P, et al. (1988) Combination of mitoxantrone and etoposide in refractory acute myelogenous leukemia--an active and well-tolerated regimen. J Clin Oncol 6: 213-217. [Crossref]
- Amadori S, Arcese W, Isacchi G, Meloni G, Petti MC, et al. (1991) Mitoxantrone, etoposide, and intermediate-dose cytarabine: an effective and tolerable regimen for the treatment of refractory acute myeloid leukemia. J Clin Oncol 9: 1210-1214. [Crossref]
- Sternberg DW, Aird W, Neuberg D, Thompson L, MacNeill K, et al. (2000) Treatment of patients with recurrent and primary refractory acute myelogenous leukemia using mitoxantrone and intermediate-dose cytarabine: a pharmacologically based regimen. Cancer 88: 2037-2041. [Crossref]
- Lancet JE, Uy GL, Cortes JE, Newell LF, Lin TL, et al. (2018) CPX-351 (cytarabine and daunorubicin) Liposome for Injection Versus Conventional Cytarabine Plus Daunorubicin in Older Patients with Newly Diagnosed Secondary Acute Myeloid Leukemia. J Clin Oncol 36: 2684-2692. [Crossref]
- Milligan DW, Wheatley K, Littlewood T, Craig JI, Burnett AK (2006) Fludarabine and cytosine are less effective than standard ADE chemotherapy in high-risk acute myeloid leukemia, and addition of G-CSF and ATRA are not beneficial: results of the MRC AML-HR randomized trial. Blood 107: 4614-4622. [Crossref]
- Brown RA, Herzig RH, Wolff SN, Frei-Lahr D, Pineiro L, et al. (1990) High-dose etoposide and cyclophosphamide without bone marrow transplantation for resistant hematologic malignancy. Blood 76: 473-479. [Crossref]
- Liu Yin JA, Wheatley K, Rees JK, Burnett AK (2001) Comparison of 'sequential' versus 'standard' chemotherapy as re-induction treatment, with or without cyclosporine, in refractory/relapsed acute myeloid leukaemia (AML): results of the UK Medical Research Council AML-R trial. Br J Haematol 113: 713-726. [Crossref]
- Liu F, Knight T, Su Y, Edwards H, Wang G, et al. (2019) Venetoclax Synergistically Enhances the Anti-leukemic Activity of Vosaroxin Against Acute Myeloid Leukemia Cells Ex Vivo. Target Oncol 14: 351-364. [Crossref]
- Ravandi F, Ritchie EK, Sayar H, Lancet JE, Craig MD, et al. (2015) Vosaroxin plus cytarabine versus placebo plus cytarabine in patients with first relapsed or refractory acute myeloid leukaemia (VALOR): a randomised, controlled, double-blind, multinational, phase 3 study. Lancet Oncol 16: 1025-1036. [Crossref]
- Wetzler M, Segal D (2011) "Omacetaxine as an anticancer therapeutic: what is old is new again". Curr Pharm Des 17: 59-64. [Crossref]
- Yu W, Mao L, Qian J, Qian W, Meng H, et al. (2013) Homoharringtonine in combination with cytarabine and aclarubicin in the treatment of refractory/relapsed acute myeloid leukemia: a single-center experience. Ann Hematol 92: 1091-1100. [Crossref]
- Chao SH, Fujinaga K, Marion JE, Taube R, Sausville EA, et al. (2000) "Flavopiridol inhibits P-TEFb and blocks HIV-1 replication". J Biol Chem 275: 28345-28348. [Crossref]
- Litzow MR, Wang XV, Carroll MP, Karp JE, Ketterling R, et al. (2014) A Randomized Phase II Trial of Three Novel Regimens for Relapsed/ Refractory Acute Myeloid Leukemia (AML) Demonstrates Encouraging Results with a Flavopiridol-Based Regimen: Results of Eastern Cooperative Oncology Group (ECOG) Trial E1906. Blood 124: 3742.
- Konopleva M, Pollyea DA, Potluri J, Chyla B, Hogdal L, et al. Efficacy and Biological Correlates of Response in a Phase II Study of Venetoclax Monotherapy in Patients with Acute Myelogenous Leukemia. Cancer Discov 6: 1106-1117. [Crossref]
- Labrador J, Saiz-Rodríguez M, de Miguel D, de Laiglesia A, Rodríguez-Medina C, et al. (2022) Use of Venetoclax in Patients with Relapsed or Refractory Acute Myeloid Leukemia: The PETHEMA Registry Experience. Cancers 14: 1734. [Crossref]
- Marcucci G, Maharry K, Wu YZ, Radmacher MD, Mrózek K, et al. (2010) IDH1 and IDH2 gene mutations identify novel molecular subsets within de novo cytogenetically normal acute myeloid leukemia: a Cancer and Leukemia Group B study. J Clin Oncol 28: 2348-2355. [Crossref]
- DiNardo CD, Stein EM, de Botton S, Roboz GJ, Altman JK, et al. (2018) Durable Remissions with Ivosidenib in IDH1-Mutated Relapsed or Refractory AML. N Engl J Med 378: 2386-2398. [Crossref]
- Intlekofer AM, Shih AH, Wang B, Nazir A, Rustenburg AS, et al. (2018) Acquired resistance to IDH inhibition through trans or cis dimer-interface mutations. Nature 559: 125-129. [Crossref]
- Stein EM, DiNardo CD, Pollyea DA, Fathi AT, Roboz GJ, et al. (2017) Enasidenib in mutant IDH2 relapsed or refractory acute myeloid leukemia. Blood 130: 722-731. [Crossref]
- Papaemmanuil E, Gerstung M, Bullinger L, Gaidzik VI, Paschka P, et al. (2016) Genomic Classification and Prognosis in Acute Myeloid Leukemia. N Engl J Med 374: 2209-2221. [Crossref]
- Daver N, Schlenk RF, Russell NH, Levis MJ (2019) Targeting FLT3 mutations in AML: review of current knowledge and evidence. Leukemia 33: 299-312. [Crossref]
- Perl AE, Martinelli G, Cortes JE, Neubauer A, Berman E, et al. (2019) Gilteritinib or Chemotherapy for Relapsed or Refractory FLT3-Mutated AML. N Engl J Med 381: 1728-1740. [Crossref]
- Cortes JE, Khaled S, Martinelli G, Perl AE, Ganguly S, et al. (2019) Quizartinib versus salvage chemotherapy in relapsed or refractory FLT3-ITD acute myeloid leukaemia (QuANTUM-R): a multicentre, randomised, controlled, open-label, phase 3 trial. Lancet Oncol 20: 984-997. [Crossref]
- Usuki K, Handa H, Choi I, Yamauchi T, Iida H, et al. (2019) Safety and pharmacokinetics of quizartinib in Japanese patients with relapsed or refractory acute myeloid leukemia in a phase 1 study. Int J Hematol 110: 654-664. [Crossref]
- Ivanoff S, Gruson B, Chantepie SP, Lemasle E, Merlusca L, et al. (2013) 5-Azacytidine treatment for relapsed or refractory acute myeloid leukemia after intensive chemotherapy. Am J Hematol 88: 601-605. [Crossref]
- Ritchie EK, Feldman EJ, Christos PJ, Rohan SD, Lagassa CB, et al. (2013) Decitabine in patients with newly diagnosed and relapsed acute myeloid leukemia. Leuk Lymphoma 54: 2003-2007. [Crossref]
- Schaefer EW, Loaiza-Bonilla A, Juckett M, DiPersio JF, Roy V, et al. (2009) A phase 2 study of vorinostat in acute myeloid leukemia. Haematologica 94: 1375-1382. [Crossref]
- Gojo I, Tan M, Fang HB, Sadowska M, Lapidus R, et al. (2013) Translational phase I trial of vorinostat (suberoylanilide hydroxamic acid) combined with cytarabine and etoposide in patients with relapsed, refractory, or high-risk acute myeloid leukemia. Clin Cancer Res 19: 1838-1851. [Crossref]
- Cortes J, Feldman E, Yee K, Rizzieri D, Advani AS, et al. (2013) Two dosing regimens of tosedostat in elderly patients with relapsed or refractory acute myeloid leukaemia (OPAL): a randomised open-label phase 2 study. Lancet Oncol 14: 354-362. [Crossref]
- Advani AS, Elson P, Kalaycio ME, Mukherjee S, Gerds AT, et al. (2014) Bortezomib+ MEC (mitoxantrone, etoposide, cytarabine) for relapsed/refractory acute myeloid leukemia: Final results of an expanded phase 1 trial. Blood 124: 978 [Crossref]
- Wei He, Caifang Zhao, Huixian Hu (2020) Prognostic effect of RUNX1 mutations in myelodysplastic syndromes: a meta-analysis. Hematology 25: 494-501. [Crossref]
- Min YH, Eom JI, Cheong JW, Maeng HO, Kim JY, et al. (2003) Constitutive phosphorylation of Akt/PKB protein in acute myeloid leukemia: its significance as a prognostic variable. Leukemia 17: 995-997. [Crossref]
- Récher C, Beyne-Rauzy O, Demur C, Chicanne G, Dos Santos C, et al. (2005) Antileukemic activity of rapamycin in acute myeloid leukemia. Blood 105: 2527-2534. [Crossref]
- Tan P, Tiong IS, Fleming S, Pomilio G, Cummings N, et al. (2016) The mTOR inhibitor everolimus in combination with azacitidine in patients with relapsed/refractory acute myeloid leukemia: a phase Ib/II study. Oncotarget 8: 52269-52280. [Crossref]
- Castaigne S, Pautas C, Terré C, Raffoux E, Bordessoule D, et al. (2012) Effect of gemtuzumab ozogamicin on survival of adult patients with de-novo acute myeloid leukaemia (ALFA-0701): a randomised, open-label, phase 3 study. Lancet 379: 1508-1516. [Crossref]
- Taksin AL, Legrand O, Raffoux E, de Revel T, Thomas X, et al. (2007) High efficacy and safety profile of fractionated doses of Mylotarg as induction therapy in patients with relapsed acute myeloblastic leukemia: a prospective study of the alfa group. Leukemia 21: 66-71. [Crossref]
- Pilorge S, Rigaudeau S, Rabian F, Sarkozy C, Taksin AL, et al. (2014) Fractionated gemtuzumab ozogamicin and standard dose cytarabine produced prolonged second remissions in patients over the age of 55 years with acute myeloid leukemia in late first relapse. Am J Hematol 89: 399-403. [Crossref]
- Ravandi F, Walter RB, Subklewe M, Buecklein V, Jongen-Lavrencic M, et al. (2020) Updated results from phase I dose-escalation study of AMG 330, a bispecific T-cell engager molecule, in patients with relapsed/refractory acute myeloid leukemia (R/R AML). Journal of Clinical Oncology 38: 7508-7508.
- Moore PA, Zhang W, Rainey GJ, Burke S, Li H, et al. (2011) Application of dual affinity retargeting molecules to achieve optimal redirected T-cell killing of B-cell lymphoma. Blood 117: 4542-4551. [Crossref]
- Uy GL, Aldoss I, Foster MC, Sayre PH, Wieduwilt MJ, et al. (2021) Flotetuzumab as salvage immunotherapy for refractory acute myeloid leukemia. Blood 137: 751-762. [Crossref]
- Walter RB, Othus M, Burnett AK, Löwenberg B, Kantarjian HM, et al. (2015) Resistance prediction in AML: analysis of 4601 patients from MRC/NCRI, HOVON/SAKK, SWOG and MD Anderson Cancer Center. Leukemia 29: 312-320. [Crossref]
- Thol F, Schlenk RF, Heuser M, Ganser A (2015) How I treat refractory and early relapsed acute myeloid leukemia. Blood 126: 319-327. [Crossref]
- Hourigan CS, Karp JE (2013) Minimal residual disease in acute myeloid leukaemia. Nat Rev Clin Oncol 10: 460-471.


