In GBM, enhancing fraction derived from the initial area under the contrast agent concentration curve (IAUC), EnFIAUC60, correlates with the MRI parameter Ktrans (contrast agent transfer coefficient). Thresholding voxels as enhancing when IAUC60>2.5mMol.s (EnFIAUC60>2.5) separates high from low grade glioma. The objectives were to evaluate the feasibility of deriving a signal intensity based enhancing fraction (EnFSI), assess its relationship with EnFIAUC60 measures and examine relationships with survival in GBM.
30 GBM had pre-operative pre and post-contrast T1-weighted imaging and a T1-DCE-MRI protocol. EnFIAUC60>0 and EnFIAUC60>2.5 were generated from IAUC60 maps. EnFSI was derived from pre and post-contrast T1-weighted sequences. Bland/Altman plots assessed agreement between EnF measures. A multivariate Cox regression analysis examined the prognostic value of EnF measures.
The mean difference between EnFIAUC60>0 and EnFSI was 0.0378 (range -0.112-0.264, std. dev 0.07573) and between EnFIAUC60>2.5 and EnFSI was -0.2492 (range -0.6096-0.2416, std. dev 0.2154). EnFSI demonstrated good correlation with both EnFIAUC60>0 and EnFIAUC60>2.5 but was not directly interchangeable with either metric. Increased EnFIAUC60>0 was associated with prolonged survival (p=0.008). A non-significant trend was seen with EnFSI (p=0.061).
EnFSI can be derived from conventional imaging and correlates with IAUC60 based metrics. Furthermore, enhancing fraction conveys potential prognostic information in GBM.
The potential of enhancing fraction (EnF) in predicting cancer outcome was first described in epithelial ovarian carcinoma where a CT based metric was found to predict clinical outcome following first line chemotherapy [1]. An MRI based measure of EnF has been shown to provide clinical information in glioma [2,3]. The commonest approach to calculating EnF from MRI is to use the initial area under the concentration time curve (IAUC60) derived from dynamic contrast enhanced MRI (DCE-MRI) and to measure the proportion of voxels within a tumour with a measurable IAUC (> 0 mMol.s) [2,3]. The application of a thresholding technique to EnF allows for the distinction between low and high grade glioma [3]. EnF has been shown to correlate with the established DCE-MRI derived parameters vp (in low grade glioma) and Ktrans (in glioblastoma multiforme, GBM)[2]. The present technique for measuring EnF requires a DCE-MRI acquisition, calculation of pixel by pixel contrast concentration time course curves providing parametric maps of IAUC60. Although IAUC60 is considered to be a more reliable, reproducible and robust measure than some pharmaco-kinetic modelling parameters such as vp [4], the requirement of a DCE-MRI acquisition and post processing analysis limits its use in the routine clinical setting.
Baseline, pre-operative measurements of blood volume (derived from both T1 DCE and DSC imaging techniques) and Ktrans (from T1 DCE) [5-11] have shown potential in predicting survival in GBM. But as stated above, these techniques can require lengthy acquisitions and complex post processing analysis, which limits their application in the clinical setting. To date, the clinical application of these metrics focuses predominantly on guiding the surgeon in targeting biopsy, evaluating for malignant de-differentiation and attempting to differentiate from treatment effect and tumour progression. In a research setting, there is some utilisation of these metrics as potential surrogate biomarkers in monitoring treatment response in clinical trials of novel therapeutic agents[12].
The aims of this study were: 1) to establish the feasibility of acquiring a measure of enhancing fraction based on changes in signal intensity (EnFSI); 2) to compare this with the more established IAUC60 (both EnFIAUC60>0 and EnFIAUC60>2.5) based technique and 3) to assess the relationship of different measurements of EnF with survival in a cohort of GBM patients.
Patients
The study had local ethical approval from the North-West Manchester Research and Ethics Committee and all patients provided written informed consent prior to imaging. All methods were performed in accordance with the relevant guidelines and regulations. Patients were identified via the weekly neuro-oncology multi-disciplinary team meetings at Salford Royal NHS Foundation Trust and inclusion criteria included patients over the age of 18 years with subsequent histologically confirmed grade IV GBM according to World Health Organisation (WHO) criteria. All imaging was performed prior to surgery. All patients received corticosteroids treatment for a minimum of 48 hours prior to imaging as the sole form of treatment prior to imaging. The minimum time period of 48 hours was chosen to ensure the steroid effects on perfusion/enhancement had plateaued by the point of imaging. Exclusion criteria included patients with contra-indication to MRI of any sort, poor renal function (contra-indicating gadolinium administration) and subsequent failure to have confirmatory diagnosis of GBM on histology.
Data acquisition
Imaging was performed on 3 Tesla Philips Achieva system (Philips Healthcare, Best, Netherlands) at the University of Manchester Magnetic Resonance Imaging Facility (Salford Royal Foundation Trust Hospital, Salford), using a SENSE 8-channel head coil. The DCE-MRI protocol images were acquired in a sagittal oblique orientation, which incorporated the internal carotid artery for measurement of an arterial input function (AIF) during the dynamic sequence and provided geometrically matched pre and post contrast T1-weighted sequences for definition of the tumour volume of interest (VOItumour_coregoblique). In addition, axial pre and post contrast T1-weighted sequences were acquired in keeping with our institution’s routine tumour imaging protocol. Sequences included: 1) An axial pre contrast T1-weighted spin echo sequence (TR 10 ms, TE 500 ms, slice thickness 4.0 mm, 256 x 256 matrix). 2) A sagittal oblique pre contrast T1-weighted sequence (TR 9.3 ms, TE 4.6 ms, slice thickness 4.2 mm, 128 x 128 matrix). 3) Three sagittal oblique pre-contrast spoiled fast field echo (T1-FFE spoiled gradient echo) sequences with different flip angles (2˚, 10˚ and 16˚) for calculation of baseline T1 maps (TR 3.5 ms, TE 1.1 ms, slice thickness 4.2 mm, 128 x 128 matrix) using the standard variable flip angle relationship [13]. 4) A sagittal oblique dynamic, contrast enhanced acquisition series with identical acquisition parameters as the variable flip angle baseline T1 measurement, consisting of 100 volumes with temporal spacing of approximately 3.4 seconds. Gadolinium-based contrast agent (Gd-DTPA-BMA; Omniscan, GE Healthcare, Oslo, Norway) was injected as a bolus of 3 ml, at 15 mls-1, at a dose of 0.1 mmolkg-1 of body weight after acquisition of the fifth image volume. 5) A sagittal oblique post contrast T1-weighted sequence (TR 9.3 ms, TE 4.6 ms, slice thickness 4.2 mm, 128 x 128 matrix). 6) An axial post contrast T1-weighted spin echo sequence (TR 10 ms, TE 500 ms, slice thickness 4.0 mm, 256 x 256 matrix)
All sagittal oblique acquisitions were co-aligned at the point of data acquisition.
Data analysis
VOIs were manually defined by an experienced radiologist (SJM) using a technique which has previously shown good inter-observer agreement (Intraclass Correlation Coefficient > 0.94) [14] VOIs (Figure 1) included a tumour VOI on the sagittal oblique post contrast T1-weighted image (VOItumour_coregoblique), a tumour VOI on the axial post contrast T1-weighted image (VOItumour_coregaxial) and a VOI of contralateral normal appearing white matter (NAWM) on the axial post contrast T1-weighted image (VOINAWM_axial). A slice including the internal carotid artery was identified by the same radiologist to allow the generation of automated arterial input functions for the DCE-MRI analysis [15]. Post processing analysis was performed using in-house software (MaDyM – Manchester Dynamic Modelling) and the extended Tofts and Kermode pharmacokinetic model [16] and parametric maps of IAUC60 were generated.
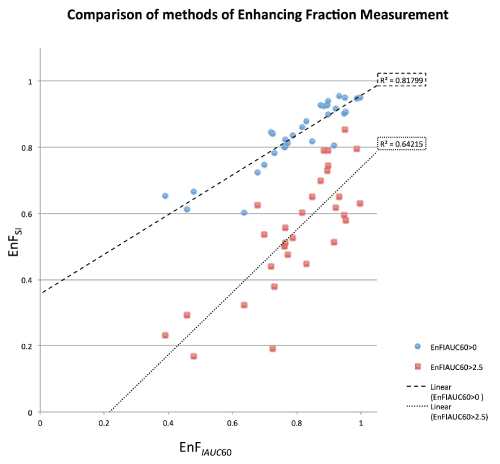
Figure 1. Scatter plot of EnFIAUC60>0 (blue circles) and EnFIAUC60>2.5 (red triangles) versus EnFSI for all patients. There is a significant correlation between EnFSI and both DCE-MRI derived parameters (EnFIAUC60>0: = 0.870, p < 0.001 and R2 = 0.818 and EnFIAUC60>2.5: = 0.744, p < 0.001 and R2 = 0.642).
MATLAB (The Mathworks, Natick, Massachusetts, USA) was used for calculating all measures of enhancing fraction. For each tumour EnFIAUC60>0 was calculated by dividing the volume of voxels with an IAUC60 > 0 mMol.s by the total volume of the VOItumour_coregoblique. EnFIAUC60>2.5 was calculated by dividing the volume of voxels with an IAUC60 > 2.5 mMol.s by the total volume of the VOItumour_coregoblique. The change in signal intensity between pre and post contrast imaging was calculated for all voxels in both VOItumour_axial and VOINAWM_axial. EnFSI was calculated by measuring the volume of voxels with enhancement greater than the mean change in signal intensity in NAWM + 2 standard deviations, divided by the total volume of voxels in the VOItumour_axial.
Statistical analysis
All statistical analysis was performed using SPSS version 15.0 (SPSS Inc., Chicago, USA). Scatter plots of EnFIAUC60>0 and EnFIAUC60>2.5 versus EnFSI were generated. Agreement between the two measures of EnF (EnFIAUC60>0 and EnFIAUC60>2.5 versus EnFSI) were assessed with Bland-Altman plots and a paired t-test on the mean observed differences between the two measures versus a mean difference of zero was performed to assess the probability of inherent bias in one method as compared with standard [17,18]. In addition, intraclass correlation analysis was performed as an alternative method of comparing the methodologies [19]. A further analysis of the relationship with all measures of EnF, age, pre-surgical tumour volume, treatment (initial chemoradiotherapy versus palliative radiotherapy) and patient survival using a multivariate Cox regression analysis was performed on a subset of patients with newly presenting GBM. Patients with recurrent GBM were excluded.
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
30 patients were included in the initial aspect of the study, comparing the different methods of EnF measurement (11 female, 19 males, mean age 59 years, age range 18-76 years). In the subsequent survival analysis, 1 patient with recurrent GBM was excluded. 1 patient was lost to follow up, but included in the analysis and censored, in accordance with standard Cox regression survival analysis.
The scatter plots for both EnFIAUC60>0 and EnFIAUC60>2.5 versus EnFSI are shown in Figure 1 and the Bland Altman plots in Figure 2. The results for the Bland Altman analysis are presented in Table 1. For EnFIAUC60>0 versus EnFSI, the paired t-test demonstrated a significant difference between the mean difference between measures and a mean difference of zero, p = 0.011. For EnFIAUC60>2.5 versus EnFSI, the paired t-test demonstrated a significant difference between the mean difference between measures and a mean difference of zero, p <0.001). Excellent intraclass correlation coefficients were demonstrated between EnFSI and both IAUC60 based measurements of EnF (EnFIAUC60>0 ICC = 0.909 and EnFIAUC60>2.5 ICC = 0.883), indicating ‘almost perfect’ consistency of between EnFSI and EnFIAUC60>0 [19]. EnFSI had a tendency to measure slightly lower than EnFIAUC60>0 and markedly higher than EnFIAUC60>2.5 (Figure 1). The lowest values of EnFIAUC60>0 (less than approximately 0.60) demonstrated the greatest discrepancy between EnFIAUC60>0 and EnFSI measures (Figure 3).
Table 1. Cox multivariate significance levels for relationship between parameters and patient overall survival. ** Significance taken as p<0.01
Parameter |
Cox Multivariate Significance Level (p=) |
Pre-operative tumour volume |
0.101 |
Age |
0.15 |
Treatment regime |
0.261 |
EnFSI |
0.061 |
EnFIAUC60>0 |
0.008** |
EnFIAUC60>2.5 |
0.384 |
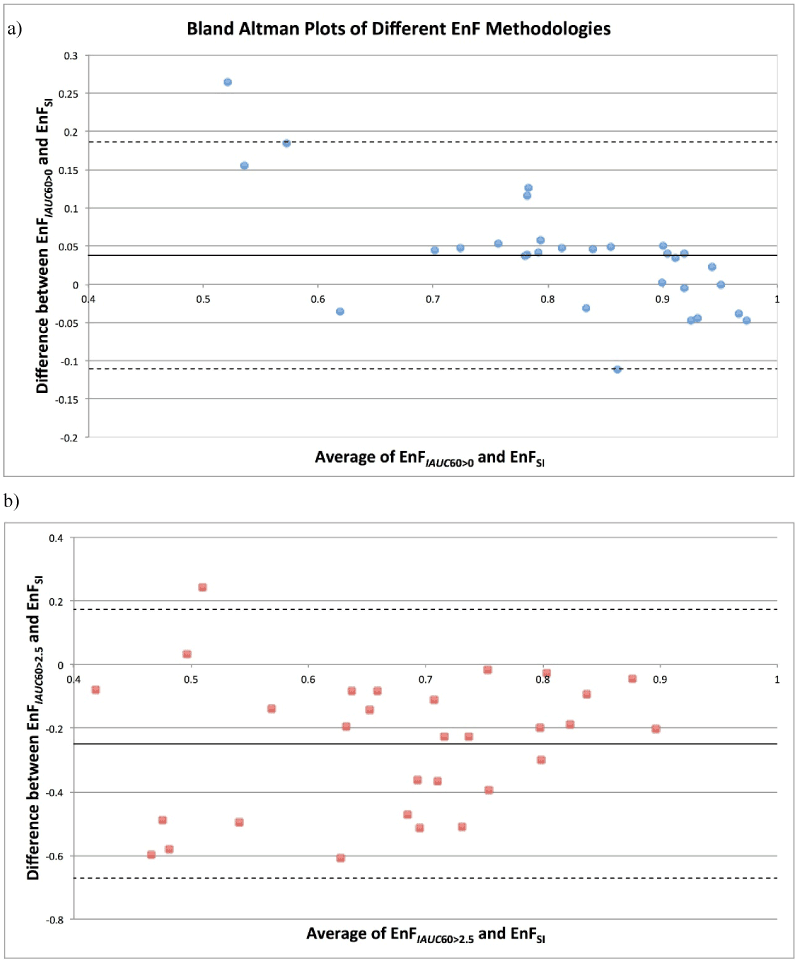
Figure 2. Bland Altman Plot of Average Enhancing Fraction determined by a) EnFIAUC60>0 and EnFSI versus the mean difference (EnFIAUC60>0 minus EnFSI) between the two measures and b) EnFIAUC60>2.5 and EnFSI versus the mean difference (EnFIAUC60>2.5 minus EnFSI) between the two measures.
Solid line = mean difference. Dashed line = + 1.96 standard deviation.
The Cox multivariable analysis results are shown in table 1. Only EnFIAUC60>0 (p=0.008) and age (p=0.01) demonstrated significant relationships with overall survival (Figure 3). A trend was seen with increasing EnFSI and patient survival, but this failed to reach significance (p = 0.061, Figure 4). Survival did not differ between patients receiving chemoradiotherapy or palliative radiotherapy (p=0.261). Neither pre-treatment tumour volume or patient age demonstrated a significant relationship with survival (p=0.101 and p=0.15, respectively).

Figure 3. Scatter plot of a) EnFIAUC60>0 and patient survival, linear regression analysis shows increased survival with increased EnFIAUC60>0 (p=0.008) and b) EnFSI and patient survival. A non-significant trend is seen with increased survival with increased EnFSI (p=0.061).
EnF is a relatively recently described parameter [2,3,20,21]. A CT variant of this measure has shown potential in predicting disease free survival and time to progression in patients with ovarian carcinoma [1]. The technique used in this study was simple, using only post contrast CT images and calculating EnF by classifying voxels as enhancing if the Hounsfield unit measure for a given voxel was greater than a set threshold. A number of patients with cerebral tumours will have a CT scan as their initial investigation, but the imaging modality of choice for brain tumour characterisation is an MRI scan and therefore the ability to derive a signal intensity based MRI measure of EnF was assessed in the current study as opposed to a CT based measure. A study of DCE-MRI derived EnF in glioma has shown the use of thresholding allows the distinction between histological grade of tumour [3]. In the unthresholded form, EnFIAUC60>0 correlated with vp in low grade tumours and Ktrans in GBM [2]. These latter studies have all required a DCE-MRI acquisition in order to derive EnF, thus limiting the translation of this research into the clinical setting. The ability to derive EnF from conventional pre and post contrast weighted imaging makes it a much more attractive parameter for clinical use. This study demonstrates that it is feasible to obtain a signal intensity based measure of EnF from routine conventional imaging, which is comparable with that derived from DCE-MRI data.
The EnF measures are not directly interchangeable; EnFSI having a tendency to measure slightly lower than EnFIAUC60>0 and markedly higher than EnFIAUC60>2.5 (Figure 1). This likely reflects the difference in thresholding between the techniques. The EnFIAUC60>0 metric has no thresholding applied at all and merely identifies a voxel as enhancing due to contrast agent being measurable. EnFSI is thresholded relative to NAWM, thereby a voxel is only considered to be enhancing if there is an increase in T1 signal intensity that is greater than would be expected in NAWM. EnFIAUC60>2.5 is thresholded to a level that provides maximum discrimination between grade II and grade IV gliomas.
Other groups have previously used signal intensity based measures to assess enhancement in glial tumours [22-24] but these have predominantly focused on assessing the intensity of enhancement, rather than the proportion of enhancement. Serial studies of such metrics have shown potential in predicting which low grade gliomas will subsequently undergo malignant transformation [24] and, in high grade gliomas, have been shown to correlate with the volume of surrounding oedema [23]. Unlike the thresholded measure of EnF (EnFIAUC60>2.5) these measures do not distinguish between histological grade of tumour [3,22]. Studies of subjective semi-quantitative [25,26] and planimetry volume [27] measures of tumour “necrosis”, defined as non-enhancement, have shown shorter survival in patients with larger necrotic volumes, and this is concordant with our findings. More recently, the change in T1 signal measured on conventional post contrast sequences on serial imaging in bevaziumab treated recurrent GBM has shown value in predicting disease progression [28].
There are potential limitations in using a signal intensity based method for calculating EnF. Unlike CT, the relationship between signal intensity and contrast agent concentration on MRI is nonlinear. However, the measurement of EnF does not require an estimation of the degree of intensity of enhancement, but merely a binary measure of whether it is present or not in order to calculate the proportion of tumour that enhances. Normalising the change in signal intensity with that seen in contralateral NAWM improves the specificity so that only changes in signal intensity greater than those seen in NAWM are classified as enhancing. This normalisation technique should also help overcome variations in signal intensity that may occur between subjects, scanners, acquisition sequences and centres.
In this study, EnFIAUC60>0 alone demonstrated a significant relationship with overall patient survival and this was irrespective of treatment, pre-operative tumour volume and age. The lack of a difference in survival between full chemoradiotherapy regime and palliative radiotherapy alone is interesting. This may reflect an unconscious bias by the clinicians to palliate patients with larger, more necrotic tumour rather than subject those individuals to a full chemoradiotherapy regime, although no relationship between pre-operative tumour volume and survival was found in our small study. A trend of increasing EnFSI and prolonged survival was noted but failed to reach significance. There are a number of differences between these two EnF measures. EnFSI is normalised to contralateral normal appearing white matter, and is therefore more likely to represent pathological ‘enhancement’ as any ‘enhancement’ similar to that seen in normal brain is excluded as ‘non-enhancing’. Due to the technique and post-processing analysis for measurement EnFIAUC60>0, no normalisation with contralateral normal appearing white matter could be made, therefore measurement of EnF via this method classifies voxels as enhancing if they contain any contrast agent within them, even within the normal vascular space (as in normal brain tissue) and therefore may give a higher measure of EnF when compared to a technique which is adjusted to normal brain tissue. Furthermore, the EnFIAUC60>0 parameter is obtained from a volumetric dataset, whilst the EnFSI is derived from non-volumetric 4 mm conventional imaging slices. No attempt was made during the conventional imaging acquisition to overcome the automatic scanner gain function and therefore variability in the measures of signal intensity may have occurred between pre and post contrast imaging for each patient, but this is unlikely to have had a significant impact thanks to the test for signal intensity enhancement difference from NAWM, which provides normalisation for changes of intensity scale. The failure for EnFSI, unlike EnFIAUC60>0, to significantly relate to survival, may reflect the small number of patients in this current study. Whilst DCE-derived EnF is likely to be a more robust and reliable parameter than EnFSI, EnFSI has the advantage of being available from conventional pre and post contrast T1-weighted sequences which are part of routine clinical imaging and do not require additional T1 mapping sequences and a DCE-acquisition and the associated complex pharmaco-kinetic post-processing making this technique a more desirable tool for the routine clinical setting.
Our findings differ from that reported by Gutman et al., in a study of 75 pre-surgical GBM, they found that visual assessment of proportional contrast enhancement showed worse survival with increasing proportional enhancement with a hazards ratio of 7.745[29]. Proportional enhancement in this study was catergorised subjectively as 0-5%, 6-33%, 34-67%, 68-95% and 95-100% by a variety of neuroradiologists, and included the surrounding oedema as part of the whole tumour volume. This differs from the work presented in this study whereby quantitative rather than categorical volumetric assessment was performed, by a single radiologist using a technique, which has previously shown good intra and inter-observer variability [14], and the surrounding T2 signal abnormality/oedema was not included in the whole tumour volume. A larger quantitative volumetric analysis of 94 GBM by the same group also reported increased proportional enhancement was associated with increased mortality (p=0.003), but as with the previously study included the perilesional T2 and FLAIR signal abnormality as part of the whole tumour volume [30]. Perez-Beteta et al. assessed the pre-treatment geometry in 117 patients with GBM and reported both increases in tumour volume and contrast enhancing volume were associated with reduced overall survival (p=0.034, hazards ratio 1.574 and p=0.017, hazards ratio 1.659), but no assessment of the proportional enhancement of the tumour was made in this study [31].
This study demonstrates that a measure of proportional enhancement can be derived from conventional pre and post contrast enhanced MR imaging. Whilst EnFSI demonstrates a significant correlation with EnF derived from DCE-MRI data, both EnFIAUC60>0 and EnFIAUC60>2.5, the two measures are not directly interchangeable. Only EnFIAUC60>0 related to patient survival, with higher values associated with prolonged survival. A similar non-significant trend was seen with the signal intensity based metric of EnF.
Both Professor Parker and Dr. Bounaccorsi have additional affiliations with the company Bioxydyn Ltd. The work presented here is unrelated to this company but the potential conflict of interest by these two co-authors is highlighted.
This work was funded as part of a Cancer Research UK Clinical Research Training Fellowship, reference C21247/A7473.
Study design was conceived by SJM, GJMP and AJ. Data analysis was performed by SJM, GT and GB. The paper was authored by SJM and AJ. All authors reviewed the paper.
- O'Connor JP, Jayson GC, Jackson A, Ghiorghiu D, Carrington BM, et al. (2007) Enhancing Fraction Predicts Clinical Outcome following First-Line Chemotherapy in Patients with Epithelial Ovarian Carcinoma. Clin Cancer Res 13: 6130-6135. [crossref]
- Mills SJ, Soh C, O'Connor JP, Rose CJ, Buonaccorsi G, et al. (2010) Enhancing Fraction in Glioma and Its Relationship to the Tumoral Vascular Microenvironment: A Dynamic Contrast-Enhanced MR Imaging Study. AJNR Am J Neuroradiol 31: 726-731. [crossref]
- Mills SJ, Soh C, O'Connor JP, Rose CJ, Buonaccorsi GA, et al. (2009) Tumour enhancing fraction (EnF) in glioma: relationship to tumour grade. Eur Radiol 19: 1489-1498. [crossref]
- Roberts C, Issa B, Stone A, Jackson A, Waterton JC, et al. (2006) Comparative study into the robustness of compartmental modeling and model-free analysis in DCE-MRI studies. J Magn Reson Imaging 23: 554-563. [crossref]
- Cao Y, Tsien CI, Nagesh V, Junck L, Haken RT et al. (2006) Clinical investigation survival prediction in high-grade gliomas by MRI perfusion before and during early stage of RT. International Journal of Radiation Oncology*Biology*Physics 64: 876-885.
- Law M, Oh S, Johnson G, Babb JS, Zagzag D, et al. (2006) Perfusion Magnetic Resonance Imaging Predicts Patient Outcome as an Adjunct to Histopathology: A Second Reference Standard in the Surgical and Nonsurgical Treatment of Low-grade Gliomas. Neurosurgery 58: 1009-1107. [crossref]
- Mills SJ, Patankar TA, Haroon HA, Balériaux D, Swindell R, et al. (2006) Do cerebral blood volume and contrast transfer coefficient predict prognosis in human glioma? AJNR Am J Neuroradiol 27: 853-858. [crossref]
- Law M, Oh S, Babb JS, Wang E, Inglese M, et al. (2006) Low-Grade Gliomas: Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging—Prediction of Patient Clinical Response. Radiology 238: 658-667. [crossref]
- Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, et al. (2008) Gliomas: Predicting Time to Progression or Survival with Cerebral Blood Volume Measurements at Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 247: 490-498. [crossref]
- Bisdas S, Kirkpatrick M, Giglio P, Welsh C, Spampinato MV, et al. (2009) Cerebral Blood Volume Measurements by Perfusion-Weighted MR Imaging in Gliomas: Ready for Prime Time in Predicting Short-Term Outcome and Recurrent Disease? AJNR Am J Neuroradiol 30: 681-688. [crossref]
- Hirai T, Murakami R, Nakamura H, Kitajima M, Fukuoka H, et al. (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 29: 1505-1510. [crossref]
- O'Connor JP, Jackson A, Parker GJ, Jayson GC (2007) DCE-MRI biomarkers in the clinical evaluation of antiangiogenic and vascular disrupting agents. Br J Cancer 96: 189-195. [crossref]
- Haase A (1990) Snapshot FLASH MRI. Applications to T1, T2, and chemical-shift imaging. Magn Reson Med 13: 77-89. [crossref]
- Thompson G, Cain JR, Jackson A, Mills SJ (2008) Interobserver agreement for cerebral glioma volumetrics on conventional MR imaging.
- Parker GJ, Jackson A, Waterton JC (2003) Automated arterial input function extraction for T1-weighted DCE-MRI.
- Tofts PS (1997) Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging 7: 91-101. [crossref]
- Bland JM, Altman DG (1986) Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1: 307-310. [crossref]
- Bland JM, Altman DG (1995) Comparing methods of measurement: why plotting difference against standard method is misleading. Lancet 346: 1085-1087. [crossref]
- Müller R, Büttner P (1994) A critical discussion of intraclass correlation coefficients. Statistics in medicine 13: 2465-2476. [crossref]
- Jayson GC, Parker GJ, Mullamitha S, Valle JW, Saunders M, et al. (2005) Blockade of Platelet-Derived Growth Factor Receptor-Beta by CDP860, a Humanized, PEGylated di-Fab', Leads to Fluid Accumulation and Is Associated With Increased Tumor Vascularized Volume. J Clin Oncol 23: 973-981. [crossref]
- Mullamitha SA, Ton NC, Parker GJ, Jackson A, Julyan PJ, et al. (2007) Phase I Evaluation of a Fully Human Anti- v Integrin Monoclonal Antibody (CNTO 95) in Patients with Advanced Solid Tumors. Clin Cancer Res 13: 2128-2135. [crossref]
- White ML, Zhang Y, Kirby P, Ryken TC (2005) Can tumor contrast enhancement be used as a criterion for differentiating tumor grades of oligodendrogliomas? AJNR Am J Neuroradiol 26: 784-790. [crossref]
- Pronin IN, Holodny AI, Petraikin AV (1997) MRI of high-grade glial tumors: correlation between the degree of contrast enhancement and the volume of surrounding edema. Neuroradiology 39: 348-350. [crossref]
- Tofts PS, Benton CE, Weil RS, Tozer DJ, Altmann DR, et al. (2007) Quantitative analysis of whole-tumor Gd enhancement histograms predicts malignant transformation in low-grade gliomas. J Magn Reson Imaging 25: 208-214. [crossref]
- Hammoud MA, Sawaya R, Shi W, Thall PF, Leeds NE (1996) Prognostic significance of preoperative MRI scans in glioblastoma multiforme. J Neurooncol 27: 65-73. [crossref]
- Lacroix M, Abi-Said D, Fourney DR, Gokaslan ZL, Shi W, et al. (2001) A multivariate analysis of 416 patients with glioblastoma multiforme: prognosis, extent of resection, and survival. Journal of Neurosurgery 95: 190-198. [crossref]
- Crawford FW, Khayal IS, McGue C, Saraswathy S, Pirzkall A, et al. (2009) Relationship of pre-surgery metabolic and physiological MR imaging parameters to survival for patients with untreated GBM. J Neurooncol 91: 337-351. [crossref]
- Ellingson BM, Kim HJ, Woodworth DC, Pope WB, Cloughesy JN, et al. (2014) Recurrent Glioblastoma Treated with Bevacizumab: Contrast-enhanced T1-weighted Subtraction Maps Improve Tumor Delineation and Aid Prediction of Survival in a Multicenter Clinical Trial. Radiology 271: 200-210. [crossref]
- Gutman DA, Cooper LA, Hwang SN, Holder CA, Gao J, et al. (2013) MR imaging predictors of molecular profile and survival: multi-institutional study of the TCGA glioblastoma data set. Radiology 267: 560-569. [crossref]
- Wangaryattawanich P, Hatami M, Wang J, Thomas G, Flanders A, et al. (2015) Multicenter imaging outcomes study of The Cancer Genome Atlas glioblastoma patient cohort: imaging predictors of overall and progression-free survival. Neuro-oncology 17: 1525-1537. [crossref]
- Pérez-Beteta J, Martínez-González A, Molina D, Amo-Salas M, Luque B, et al. (2017) Glioblastoma: does the pre-treatment geometry matter? A postcontrast T1 MRI-based study. Eur Radiol 27: 1096-1104. [crossref]



