Cancer is still a serious killer globally; it is ranked the second leading cause of death after Ischaemic heart disease and stroke. According to recent world statistics, there was 8.7 million cancer death in 2015 with 17.5 million new cancer cases [1]. Between 2005 and 2015, cancer cases increased by 33%. However, a considerable decline in cancer mortality at least in the US, over the past 2 decades because of steady reductions in smoking and advances in early detection and treatment, for the 4 major cancers, lung, breast, prostate, and colorectal [2,3]. According to WHO report in 2017 http://www.who.int/mediacentre/factsheets/fs297/en/, 30–50% of cancers can currently be prevented by avoiding risk factors and implementing existing established prevention strategies. Cancer burden can be reduced by early detection of cancer and management of cancer patients. Several cancers could be cured if diagnosed early and treated effectively.
Benign and non-malignant tumors in general, are usually non-life-threatening, slow growing and do not metastasize in this stage. There are several types of common benign and non-malignant tumors, such as meningiomas, fibroma, lipoma, chondroma, osteoma, myoma, hemangioma, lymphangioma and adenoma [4]. No specific cause has been confirmed for benign and non-malignant tumors but speculations include that include genetic and various environmental factors have been suggested. Since most of our knowledge about tumors are largely based on the study of malignant tumors, that necessitate to complement such studies by studying benign and non-malignant tumors to have a better perspective and understanding of the possible factors responsible for tumor formations and growth from factors that might be responsible for malignancy-transformation.
Meningiomas are the most frequently reported intracranial tumors and other CNS tumors by histology (36.6%) [5]. Most meningiomas are non-malignant, WHO grades I and II (81.1% and 16.9% respectively) and malignant meningioma, WHO grade III, is about 1.7%. Meningiomas originate from the outermost layer of the meninges, of the arachnoid (cap cells) that are non-neuroepithelial. These cap cells are a morphologically distinct and biochemically active subgroup of arachnoidal cells [6].
Recently, it became clear that the two epigenetic modifications, microRNAs and DNA methylation are the most critical players in the regulation of gene expression including cancer and tumorigenesis [7,8]. A deep sequencing study involving small RNA libraries of non-malignant meningiomas in comparison to that of normal dura controls was performed, after the approval of the University of Tromsø Ethical Committee (REK Nord). After subsequent validation by RT-qPCR in more patient samples and more normal controls., we found a significant differential expression of some microRNAs [9,10]. The tumor suppressors miR-143, miR-193b, miR-451 were under-expressed relative to normal dura controls suggesting pro-proliferation of the tumors. On the other hand, tumor suppressors, miR-34a and miR-218 are overexpressed while the oncogenic miR-21 was under-expressed suggesting strong barriers against progression to malignancy. Moreover, some selected putative targets mRNAs were also differentially expressed between the non-malignant meningiomas and dura controls by RT-qPCR and with validation by immunohistochemistry (IHC) in tumors and arachnoid from cadavers. Like the miRNA picture, the pro-proliferation p63 and cyclinD1 were overexpressed coupled with under expression of the tumor suppressor RUNX1T1 in tumors relative to normal meninges controls, while tumor suppressor PTEN and E-Cadherins (CDH1) are overexpressed. This is noting that only 10 mRNA selected putative targets were studied for expression status. A complete mRNA expression profile will further clarify the picture that is in addition to possible mechanistic studies using animal models. However, this study of non-malignant meningiomas suggests the expression of factors promoting tumor formation and growth, but also, the expression of factors succeeded in preventing malignant progression and enforced the predominant phenotype. The questions here are: 1. Are the same or similar factors involved in all non-malignant tumors?, 2. Would tumor profiling helps in a more accurate diagnosis and prognosis, 3. Would such results leads to the development of therapeutics based on inhibiting (antisense, antibodies, chemical molecules) of the factors that are pro-proliferation or replenish the low level tumor suppressors even in malignant tumors, 4. would introducing factors that are anti-malignant progression have any therapeutic value. As always more research is needed (Figure 1).
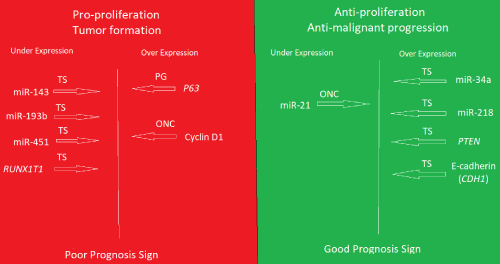
Figure 1. Summary of the expression profile of the non-malignant meningiomas grades I and II in comparison to normal meninges controls
The lower level of expression of the tumor suppressors (TS) miR-143, miR-193b, miR-451, and RUNX1T1 mRNA, coupled with higher level expression of the oncogene (ONC) Cyclin D1 and the Pro-growth (PG). would at least promote the formation and growth of the tumors. Conversely, the lower level of miR-21 (ONC) and the higher level of tumor suppressors (TS), miR-34a, miR-218, PTEN and E-cadherin (CDH1) likely, prevented the malignancy progression (dominant phenotype). The expression status of miR-143, miR-193b and RUNX1T1 in meningioma was reported for the first time in this work.
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Allen C, Barber RM, Barregard L, et al. (2017) Global, Regional, and National Cancer Incidence, Mortality, Years of Life Lost, Years Lived With Disability, and Disability-Adjusted Life-years for 32 Cancer Groups, 1990 to 2015: A Systematic Analysis for the Global Burden of Disease Study. JAMA Oncol 3: 524-548. [Crossref]
- Siegel RL, Miller KD, Jemal A (2017) Cancer Statistics, 2017. CA Cancer J Clin 67: 7-30. [Crossref]
- Jemal A, Thun MJ, Ries LA, Howe HL, Weir HK, et al. (2008) Annual report to the nation on the status of cancer, 1975-2005, featuring trends in lung cancer, tobacco use, and tobacco control. J Natl Cancer Inst 100: 1672-1694. [Crossref]
- Kumar V, Abbas Abul K, Fausto, Aster JC (2009) Robbins and Cotran Pathological basis of disease, (8th edn), 263: Saunders Elsevier.
- Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, et al. (2016) CBTRUS Statistical Report: Primary Brain and Other Central Nervous System Tumors Diagnosed in the United States in 2009-2013. Neuro 18: v1-v75. [Crossref]
- Marosi C, Hassler M, Roessler K, Reni M, Sant M, et al. (2008) Meningioma. Crit Rev Oncol Hematol 67: 153-171. [Crossref]
- Wang S, Wu W, Claret FX (2017) Mutual regulation of microRNAs and DNA methylation in human cancers. Epigenetics 12: 187-197. [Crossref]
- Markopoulos GS, Roupakia E, Tokamani M, Chavdoula E, Hatziapostolou M, et al. (2017) A step-by-step microRNA guide to cancer development and metastasis. Cell Oncol (Dordr) 40: 303-339. [Crossref]
- El-Gewely MR, Andreassen M, Walquist M, Ursvik A, Knutsen E, et al. (2016)-a Differentially Expressed MicroRNAs in Meningiomas Grades I and II Suggest Shared Biomarkers with Malignant Tumors. Cancers (Basel) 8. [Crossref]
- El-Gewely MR, Hennig R, Johansen SD (2016)-b Lessons learned from the study of non cancerous meningioma tumors. [https://atlasofscience.org/lessons-learned-from-the-study-of-non-cancerous-meningioma-tumors/]

