Na+/K+ pump is a fundamental metabolic mechanism in cell membrane which controls cell functional activity. It generates Na+ gradient on cell membrane and serves as an energy source for a number of secondary ion transporters in membrane, such as Na+/Ca2+ and Na+/H+ exchangers, Na+/sugars, amino acids and different osmolytes [1]. It is known that Na+/K+ pump, with the function of controlling intracellular ionic homeostasis, works in electrogenic regime, generates the metabolic component of membrane potential [2-4] and has a crucial role in cell volume regulation [5,6]. The activation of Na+/K+ pump leads to generation of water efflux from the cells by a) push out of 3Na+ and uptake of 2K+ and b) release of H2O in cytoplasm (42 H2O for one molecule glucose oxidation) as a result of activation of intracellular oxidative phosphorylation [7]. Such a Na+/K+ pump-induced water efflux has a great physiological meaning as it balances the osmotic water uptake [5] by cell and inactivates Na+ and Ca2+ inward currents through the membrane [8,9].
We have previously shown that Na+/K+ pump-dependent regulation of cell volume is a powerful metabolic mechanism through which both the auto-regulation of Na+/K+-pump [10] and the regulation of membrane chemosensitivity [11] and excitability [8] are realized by changing surface-dependent number of functionally active proteins in membrane.
It is known that the dysfunction of Na+/K+ pump, which is accompanied by the increase of intracellular Ca2+ concentration ([Ca2+]i), is a common consequence of any cell pathology (including aging). Traditionally, the increase of [Ca2+]i, which is accompanied by Na+/K+ pump inactivation, is considered as a result of intracellular Na+ concentration ([Na+]i) increase which stimulates Ca2+ uptake through Na+/Ca2+ exchange in reverse mode (R Na+/Ca2+ exchange) [12]. However, our previous study has shown that cGMP and cAMP modulate Na+/Ca2+ exchange activity without significantly changing [Na+]i [13-15]. The factors, which elevate intracellular cGMP, lead to activation of Na+/Ca2+ exchange in forward mode (F Na+/Ca2+ exchange) [15-18], while the factors, having elevation effect on cAMP content, activate R Na+/Ca2+ exchange (Na+ efflux and Ca2+ influx) [19-21]. From these data it can be concluded that cGMP/cAMP-dependent Na+/Ca2+ exchange, which has a crucial role in [Ca2+]i control, is more sensitive to environmental factors than Na+/K+ pump activity. Considering the fact that [Ca2+]i is a strong inhibitor for Na+/K+-ATPase [22], it is suggested that [Ca2+]i increase precedes the dysfunction of Na+/K+ pump in cell pathology.
The data presented in Figure 1 indicates that in normal physiological solution mechanical vibration and pulsing magnetic field, with the activation effect on F Na+/Ca2+ exchange, inhibit Ca2+ uptake; while in K+-free physiological solution, when pump is depressed and brings to elevation of intracellular cAMP, the same factors activate R Na+/Ca2+ exchange [23].
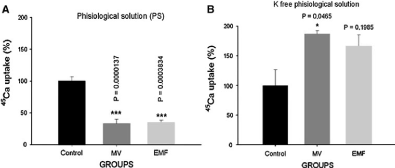
Figure 1: The effects of MV on 45Ca2+ uptake by neuronal ganglia in normal (A) and K+-free PS (B) [23].
From these data, it can be concluded that the factors, which activate cGMP-dependent F Na+/Ca2+ exchange in healthy organism, can also stimulate cAMP-dependent R Na+/Ca2+ exchange in cell pathology, when pump is depressed.
At present, it is established that Na+/K+-ATPase in somatic cells of mammals have three catalytic isoforms with different affinities to ouabain (specific inhibitor for Na+/K+-ATPase) and various functions: low (α1 isoform), middle (α2 isoform) and high (α3 isoform) affinities [24]. Among these receptors, α3 isoform is not directly involved in Na+/K+ pumping process and has only signaling function [25].
Our earlier study performed on Helix snail neurons has shown that, similar to mammalian cells, in these neuronal membranes there are three families of ouabain receptors with different affinities. The curve of dose-dependent 3[H]-ouabain binding with membrane consists of three components - two saturated (10-10-10-9M and10-8-10-7M) and one linear (10-7-10-3M). Among these three families of receptors, only the receptors with dose-dependent linear character have Na+/K+ pump function. As can be seen in Figure 2, ouabain at >10-7M concentrations has inactivation effect on Na+ efflux (Na+/K+ pump), while <10-7M ouabain concentrations have activation effects on 22Na+ efflux from the cells (Figure 2A) [10].
This activation effect on 22Na+ efflux takes places without significantly changing Na+/86Rb+ pump activity (where K+ was replaced by 86Rb+) (Figure 2B). Na+/K+ pump-independent 22Na+ efflux is activated by the increase of extracellular Ca2+ concentration, which is due to Na+ exchange with 45Ca2+ uptake (Figure 3) [26]. It is interesting to note that in pioneer work of Prof. Baker and his co-authors on fundamental study of Na+/Ca2+ exchange, which was performed on internally perfused squid axon, ouabain-sensitive (Na+/K+ pump) and ouabain-insensitive (Na+/Ca2+ exchange) components of 22Na+ efflux were identified [12]. Thus, the absence of low ouabain-induced activation of Na+/Ca2+ exchange in internally perfused axon (which is present in intact neurons) indicates the involvement of a cytoplasmic mechanism(s) in ouabain-induced activation of R Na+/Ca2+ exchange.
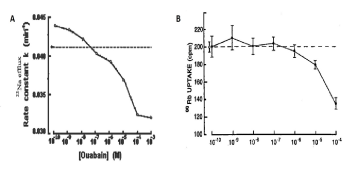
Figure 2: A. The rate constant as a function of the concentration of ouabain in the medium. The dashed line indicates the rate constant in normal Ringer’s solution. The values of the rate constant are equivalent to the values at 5 min. The limits of ±SE do not exceed the diameter of the symbols [10]. B. 86Rubidium uptake as a function of ouabain concentration. Results shown are the means of ±SEM of ten experiments, each period from study of ten pooled ganglia. The open circle at the left represents 86Rb uptake in the absence of ouabain, and this level is indicated in the dashed line [14].
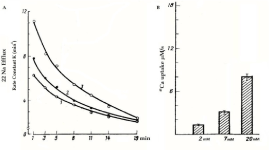
Figure 3: A. The rate constant of 22Na+ efflux as a function of ouabain concentration in K+-free physiological solution containing 2 (1), 7 (2) and 20 (3) mM Ca2+. B. 45Ca2+ uptake in K+-free medium containing 2, 7 and 20 mM Ca2+ [26].
The study of dose-dependent 3[H]-ouabain binding with neuronal membrane in isotonic, hypertonic and hypotonic solutions has shown, that compared with the number of ouabain receptors in cell membrane incubated in isotonic solution, both the numbers of pump units (α1 isoforms) and ouabain receptors responsible for activation of R Na+/Ca2+ exchange (α2/α3 isoforms) are higher in hypotonic and lower in hypertonic solutions (Figure 4) [10]. It is notable that the osmosensitivity of high affinity ouabain binding sides is more pronounced that of high affinity ones (>10-7M).
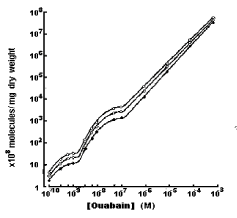
Figure 4: Binding of [3H] ouabain to Helix pomatia cell membranes as a function of the concentration of glycoside in solutions with different tonicities. Binding was measured after 2 hr of exposure to [3H] ouabain at 22oC (O) Cells exposed to glycoside in hypotonic solution (0mM sucrose; Ƭi=0.5); (○) cells exposed in isotonic solutions (63mM surcose; Ƭi=1); (●) cells exposed in hypertonic solutions (189mM surcose; Ƭi=2). Both axes are logarithmic [10].
It is known that the differences of electrochemical gradients of Na+ and Ca2+ serve as energy sources for Na+/Ca2+ exchange [12]. Traditionally, both low and high ouabain concentrations-induced stimulations of Na+/Ca2+ exchange are explained by Na+/K+ pump inhibition leading to the increase of [Na+]i [24]. However, as the above presented data indicate, low concentrations of ouabain stimulate Na+/Ca2+ exchange without notable changes in Na+/K+ pump activity [14]. As the activities of Na+/Ca2+ exchange and Na+/K+ pump are realized by different proteins, it is suggested that the close-talking correlation between them can be realized not only by the changes of [Na+]i but also by the changes of [Ca2+]i. It is obvious that [Ca2+]i can be decreased through [Ca2+]i sorption by intracellular structure and [Ca2+]i dilution by increasing intracellular water contents.
As it is noted above, low ouabain-induced activation of R Na+/Ca2+ exchange is accompanied by the increase of intracellular cAMP content in neurons [14]. It has also been shown that the activation of R Na+/Ca2+ exchange, which is accompanied by the increase of intracellular cAMP, takes place upon the effect of extremely low concentrations of biologically active substances (such as synaptic transmitters, H2O2) [27,28] and physical factors (such as background radiation, microwave with non-thermal intensity) [16,23] which are unable to activate ionic channels in membrane [16]. There is a great number of literature data showing that nM ouabain elevates intracellular cAMP content in different tissues, including dog renal cortex, goldfish intestinal mucosa, mouse pancreatic islets, murine epithelioid and fibroblastic cell lines, rat brain, rat renal collecting tubule cells in culture and astrocytes [29]. It has been shown that intracellular cAMP has activation effect on Ca2+ pump localized in endoplasmatic reticulum (ER) membrane transporting Ca2+ from cytoplasm into ER [30]. It is known that Na+/Ca2+ exchange functions in stoichometry of 3Na+:1Ca2+ [12]. Therefore, it is predicted that the activation of R Na+/Ca2+ exchange should bring to cell dehydration. However, by our previous study performed on brain and heart muscle tissues of young (healthy) rats it has been shown that nM ouabain-induced elevation of intracellular cAMP leads to activation of R Na+/Ca2+ exchange, which is accompanied by cell hydration. It has also been shown that both the rate of R Na+/Ca2+ exchange and cell hydration have a metabolic nature and age-dependent weakening, reverse character [19,31].
Thus, based on the aforementioned data, it can be suggested that in healthy animals there are minimum two mechanisms through which cAMP activates R Na+/Ca2+ exchange as a result [Ca2+]i decrease. They are a) the activation of Ca2+ pump in ER membrane and b) increase of intracellular water content leading to dilution of [Ca2+]i. However, in old and unhealthy animals Na+/K+ pump dysfunction increases [Na+]i, which in its turn stimulates R Na+/Ca2+ exchange bringing to the increase of [Ca2+]i. As the latter has multi-poisoning effect on cell metabolism, the activation of R Na+/Ca2+ exchange is accompanied by cell dehydration. Therefore, it is suggested that R Na+/Ca2+ exchange-induced cell dehydration can be considered as a primary mechanism for cell pathology.
- Lang F, Hoffmann EK (2013) CrossTalk proposal: Cell volume changes are an essential step in the cell death machinery. J. Physiol 591: 6119-6121. [Crossref]
- Ayrapetyan SN (1969) Metabolically dependent fraction of membrane potential and electrode properties of the membrane of giant neurons in mollusks. Biofizika 14: 1027-1031. [Crossref]
- Gorman ALF, Marmor MF (1970) Contributions of the sodium pump and ionic gradients to the membrane potential of a molluscan neurone. J. Physiol 210: 897-917. [Crossref]
- Thomas RC (1972) Electrogenic sodium pump in nerve and muscle cells. Physiol. Rev 52: 563-594. [Crossref]
- Ayrapetyan SN, Sulejmanyan MA (1979) On the pump-induced cell volume changes. Comp. Biochem. Physiol., part A Comp. Pharmacology 64: 571-575. [Crossref]
- Carpenter DO, Fejtl M, Ayrapetyan SN, Szarowski DH, Turner JN (1992) Dynamic changes in neuronal volume resulting from osmotic and sodium transport manipulations. Acta Biologica Hungarica 43: 39-48. [Crossref]
- Lehninger AL (1970) Mitochondria and calcium ion transport. Biochem J 119:129-138. [Crossref]
- Ayrapetyan SN, Rychkov GY, Suleymanyan MA (1988) Effects of water flow on transmembrane ionic currents in neurons of Helix pomatia and in squid giant axons. Comp Biochem Physiol A Comp Physiol 89: 179-186. [Crossref]
- Ayrapetyan SN, Rychkov GY, Suleymanian MA (1988) Modulation of calcium currents in the snail neuron membrane by osmotic gradient. Dokl Akad Nauk SSSR 300: 983-985. [Crossref]
- Ayrapetyan SN, Suleymanyan MA, Saghyan AA, Dadalyan SS (1984) Autoregulation of the electrogenic sodium pump. Cell Mol Neurobiol 4: 367-383. [Crossref]
- Ayrapetyan SN, Arvanov VL, Maginyan SB, Azatyan KV (1985) Further study of the correlation between Na-pump activity and membrane chemosensitivity. Cell Mol Neurobiol 5: 231-243. [Crossref]
- Baker PF, Blaustein MP, Hodgkin AL, Steinhardt RA (1969) The influence of calcium on sodium efflux in squid axons. J Physiol 200: 431-458. [Crossref]
- Azatyan KV, Karapetyan IC, Ayrapetyan SN (1994) Effect of Acetylcholine of low doses on 45Ca influx into the Helix Pomatia neurons. Biol. Mem. Harwood Academic Publishers GmbH 7: 301-304 (reprinted in the United States). [Crossref]
- Saghian AA, Ayrapetyan SN, Carpenter DO (1996) Low concentrations of ouabain stimulate Na/Ca exchange in neurons. Cell Mol Neurobiol 16: 489-498. [Crossref]
- Azatian KV, White AR, Walker RJ, Ayrapetyan SN (1998) Cellular and molecular mechanisms of nitric oxide-induced heart muscle relaxation. Gen. Pharmac: The Vascular System 30: 543-553. [Crossref]
- Ayrapetyan S (2012) Cell hydration as a universal marker for detection of environmental pollution. Environmentalist J 32: 210-221. [Crossref]
- Ayrapetyan G, Papanyan A, Hayrapetyan H, Ayrapetyan S (2005) Metabolic pathway of magnetized fluid-induced relaxation effects on heart muscle. Bioelectromagnetics 26: 624-630. [Crossref]
- Dadasyan E, Ayrapetyan G, Baghdasaryan N, Mikayelyan Y, Ayrapetyan S (2012) The metabolic pathway of 4Hz mechanical vibration-induced effect on snail heart muscle contractility. Environmentalist 32: 166-174. [Crossref]
- Narinyan L, De J, Ayrapetyan S (2014) Age-dependent increase in Ca2+ exchange magnetosensitivity in rat heart muscles. Biochemistry and Biophysics 2. [Crossref]
- Heqimyan A, Narinyan L, Nikoghosyan A, Ayrapetyan S (2015) Age-dependent magnetic sensitivity of brain and heart muscles. In: Markov M (Ed.) Electromagnetic Fields in Biology and Medicine, USA, CRC Press, pp: 217-230. [Crossref]
- Ayrapetyan SN (2015) The role of cell hydration in realization of biological effects of non-ionizing radiation (NIR). Electromagnetic biology and medicine 34: 197-210. [Crossref]
- Skou J (1957) The influence of some cations on an adenosine triphosphatase from peripheral nerves. Biochim Biophy Acta 23: 394-401. [Crossref]
- Ayrapetyan S, Baghdasaryan N, Mikayelyan Y, Barseghyan S, Martirosyan V, et al. (2015) Cell hydration as a marker for nonionizing radiation. In: Markov M (Ed.) Electromagnetic Fields in Biology and Medicine, USA, CRC Press, pp: 193-215. [Crossref]
- Blaustein MP, Lederer WJ (1999) Na+/Ca2+ exchange. Its physiological implications. Physiol Rev 79: 763-854. [Crossref]
- Xie Z, Askari A (2002) Na(+)/K(+)-ATPase as a signal transducer. Eur J Biochem 269: 2434-2439. [Crossref]
- Saghyan AA (1991) The ouabain-insensitive fraction of sodium efflux from Helix pomatia neurons. Biological membranes 8: 711-718 (in Russian). [Crossref]
- Ayrapetyan SN, Carpenter DO (1991) The modulatory effect of extreamly low doses of mediators on functional activity of the neuronal membrane. Zh Evol Biokhim Fiziol 27: 146-151 (in Russian). [Crossref]
2021 Copyright OAT. All rights reserv
- Dadalyan SS, Kiss T, Azatian KV, Ayrapetyan SN, Salanki J (1988) Effect of low concentration of GABA on the ACh sensitivity of snail neurons. In: Salanki J (Ed.) Neurobiology of Invertebrates, Budapest, pp: 643-653. [Crossref]
- Siegel GJ (1999) Basic Neurochemistry (6th edn) : Molecular, Cellular and Medical Aspects. Philadelphia, Lippincott-Raven, p. 1183. [Crossref]
- Brini M, Carafoli E (2009) Calcium pumps in health and disease. Physiol. Rev 89: 1341-1378. [Crossref]
- Heqimyan A, Narinyan L, Nikoghosyan A, Deghoyan A, Yeganyan A (2012) Age dependency of high affinity ouabain receptors and their magneto sensitivity. The Environmentalist 32: 228-235. [Crossref]




