Multiple myeloma, a plasma cell neoplasm commonly targets bone, bone marrow, and the kidneys. We describe a 44-year-old female patient who presented with progressive weakness of left upper limb proceeding from distal towards proximal aspect over 3 months. The patient was diagnosed as immunoglobulin G (IgG) kappa multiple myeloma with plasmacytoma complicated with brachial plexus compression at C2 to C7 levels. The patient was treated with high dose steroids, palliative radiotherapy followed by bortezomib, lenalidomide and dexamethasone. Her disease progressed while on treatment and she succumbed to the illness three months later. Plasmablastic myeloma with brachial plexopathy as the sole initial manifestation is extremely rare and this possibility should be considered in the differentials in such presentation.
multiple myeloma in young, plasmablastic myeloma, neuropathy, brachial plexopathy
Plasmablastic myeloma (PBM) is a morphologic subset of myeloma with bone marrow (BM) aspirate slide stained with Wright's stain showing ≥ 2% of plasmablasts [1]. The prevalence of plasmablastic morphology in newly diagnosed myeloma patients is approximately 10%. In multiple myeloma, peripheral neuropathy may be due to the plasma cell dyscrasia (particularly in POEMS [polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, skin changes] syndrome), or by direct compression (radicular or medullary), light chain deposits (amyloidosis), cryoglobulinemia or by autoimmune mechanism. Here, we report a case of a 44- year-old female, who was diagnosed to have plasmablastic myeloma. She presented with progressive weakness of the left upper limb and was detected to have plasmacytoma of cervical vertebra which was causing brachial plexopathy. She was treated with steroids, palliative radiotherapy and multi-agent chemotherapy but her disease progressed while on chemotherapy and she succumbed to the illness due to renal failure.
A 44-year old female was in good health until she experienced progressive weakness of the distal aspect of her left upper limb. Her symptoms gradually progressed proximally towards her left shoulder over the next three months. She had no associated bowel or bladder complaints. At presentation, her Eastern Cooperative Oncology Group score was 3. Neurological examination demonstrated features of motor and sensory weakness in left upper limb from C5 to C8 level. MRI of the cervical spine showed an enhancing soft tissue mass noted in the left paravertebral region at C2 to C7 levels with involvement of the left side of vertebral body and transverse processes and seen extending intraspinally at these levels without compressing the spinal cord (Figure 1). CT cervical spine showed 6.4 × 4 × 2.5 cm homogeneous enhancing mass lesion in the left perivertebral space with destruction of the transverse process of C4, C5 and C6 vertebrae. Routine laboratory studies revealed hemoglobin 10.2 g/dl with an ESR of 120 mm/hr. Rouleaux formation was seen on peripheral blood smear. Trucut biopsy obtained from the lesion at the cervical level revealed sheets of plasma cells having vacuolated cytoplasm with hyperchromatic round/oval nucleus. Immnohistochemical stain demonstrated that tumour cells were positive for CD38, MUM1, kappa light chain and negative for LCA (CD45), CD20, Tdt (terminal deoxynucleotidyl transferase), CD79a, PAX5, Alk with MIB1 labelling index around 90%. Histopathology and immunohistochemisry was confirmatory of plasmablastic myeloma. Skeletal survey demonstrated diffuse lytic lesions in multiple bones. Serum protein profile showed altered albumin-globulin ratio, elevated serum immunoglobulins, elevated IgG (4647 mg/dl) and free kappa light chain (1727 mg/dl) with high free kappa/lambda ratio (116.7) and immune fixation electrophoresis confirmed IgG kappa monoclonal gammopathy. Bone marrow examination revealed 60% plasma cell compatible with a diagnosis of multiple myeloma. Urine was positive for Bence-Jones protein and her 24 h urinary protein excretion was 1739 mg/day. Serum beta 2-microglobulin was 12.3 mg/L. The final diagnosis was immunoglobulin G kappa multiple myeloma with plasmablastic plasmacytoma complicating brachial plexopathy. She was administered pulse steroids (dexamethasone 40 mg IV for four days), palliative radiation and her symptoms improved minimally. She was started on bortezomib, lenalidomide and dexamethasone chemotherapy. (Bortezomib 2 mg IV on days 1, 8, 15 and 22 plus lenalidomide 20 mg PO daily on days 1–21 plus dexamethasone 40 mg PO daily on days 1, 8, 15, and 22; Q 28 d cycle). Her disease progressed (both clinically and biochemically) while on chemotherapy and she succumbed to the illness three months later due to renal failure.
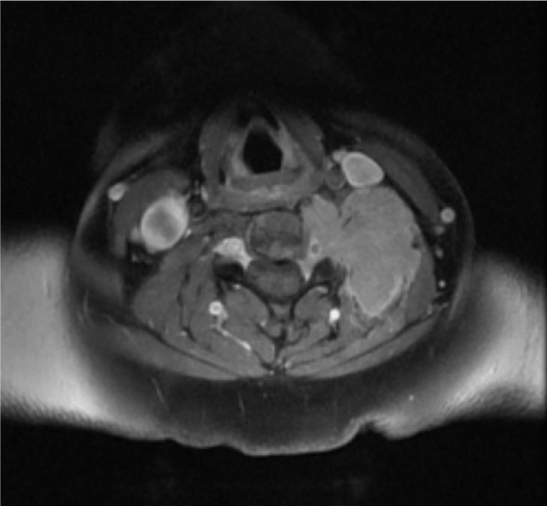
Figure 1. MRI of the cervical spine (axial view) shows a soft tissue mass noted in the left paravertebral region at cervical level with involvement of the left side of vertebral body and transverse processes and seen extending intraspinally at these levels without compressing the spinal cord
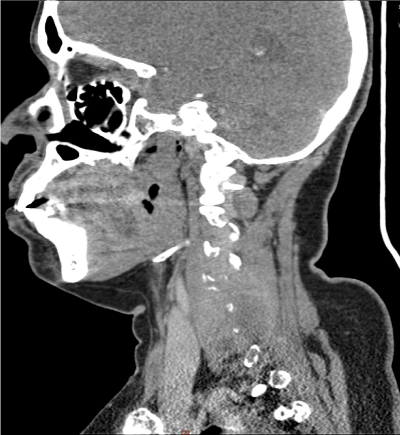
Figure 2. CT of the cervical spine (sagittal view) shows homogeneous mass lesion noted in the left perivertebral space with destruction of the transverse process of C4, C5 and C6 vertebrae
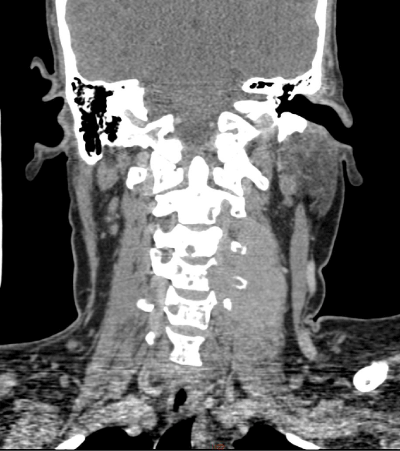
Figure 3. CT of the cervical spine (coronal view) shows homogeneous mass lesion noted in the left perivertebral space with destruction of the transverse process of C4, C5 and C6 vertebrae
Plasma cell myeloma has a broad cytologic spectrum, ranging from small, lympho-plasmacytic variant and mature plasma cells to large cells with prominent central nucleoli and immature cytoplasm, commonly referred as plasmablasts. Plasmablastic myeloma is a morphologic subset of myeloma defined by presence of ≥ 2% plasmablasts in the bone marrow aspirate [1]. Plasmablastic myeloma represents 5-15% of the cases of multiple myeloma [2] and has an independent prognostic factor predicting shorter overall survival. Median overall survival of myeloma patients with plasmablastic morphology is 1.9 years [3]. Thus, plasmablastic morphologic feature confers a poor clinical prognosis and early identification is useful in predicting aggressive clinical behaviour. Two close differentials of plasmablastic myeloma are plasmablastic lymphoma and high-grade extramedullary plasmacytoma which show significant morphologic and immunophenotypic overlap with plasmablastic myeloma. Certain clinical features like osteolytic lesions, diffuse bone marrow involvement, and the presence of an M protein favour the diagnosis of myeloma whereas, features like involvement of oral cavity, HIV and Epstein-Barr virus association favour plasmablastic lymphoma. However, few cases with atypical clinical features can pose significant diagnostic challenges. Plasmablastic myeloma, regardless of its extensiveness or localisation, should be treated as high-risk myeloma [4]. Autologous stem cell transplantation is the standard of care for young high-risk multiple myeloma patients who respond to conventional myeloma chemotherapy [4-6]. Among the myeloma subtypes, plasmablastic myeloma has been reported to have poor prognosis with increased risk of complications, relapse and refractory disease [3,4,7]. Though the association between peripheral neuropathy and multiple myeloma is well documented, it occurs only in about 3% of patients. However, peripheral neuropathy is observed in about 50% of patients with solitary plasmacytomas [8,9]. The peculiarity of peripheral neuropathy associated with plasmacytomas is that motor weakness is more prominent than sensory loss [9]. The motor weakness usually begins distally and spreads proximally in a symmetrical pattern [9]. Recently a similar case of brachial plexopathy in multiple myeloma was reported by Fukunaga H et al., where extramedullary neural involvement was demonstrated by FDG-PET scan [10].
- Greipp PR, Raymond NM, Kyle RA, O’Fallon WM (1985) Multiple myeloma: Significance of plasmablastic subtype in morphological classification. Blood 65: 305-310. [Crossref]
- Lorsbach RB, Hsi ED, Dogan A, Fend F (2011) Plasma cell myeloma and related neoplasms. Am J Clin Pathol 136: 168-182. [Crossref]
- Greipp PR, Leong T, Bennett JM, Gaillard JP, Klein B, et al. (1998) Plasmablastic morphology: An independent prognostic factor with clinical and laboratory correlates: Eastern Cooperative Oncology Group (ECOG) Myeloma Trial E9486 report by the ECOG Myeloma laboratory group. Blood 91: 2501-2507. [Crossref]
- Albarracin F, Fonseca R (2011) Plasma cell leukemia. Blood Rev 25: 107-112. [Crossref]
- Palumbo A, Anderson K (2011) Multiple myeloma. N Engl J Med 364: 1046-1060. [Crossref]
- Raab MS, Podar K, Breitkreutz I, Richardson PG, Anderson KC (2009) Multiple myeloma. Lancet 374: 324-339. [Crossref]
- Rajkumar SV, Fonseca R, Lacy MQ, Witzig TE, Therneau TM, et al. (1999) Plasmablastic morphology is an independent predictor of poor survival after autologous stem-cell transplantation for multiple myeloma. J Clin Oncol 17: 1551-1557. [Crossref]
- Silverstein A, Doniger DE (1963) Neurologic complications of myelomatosis. Arch Neurol 9: 534-544. [Crossref]
- Kelly JJ Jr., Kyle RA, Miles JM, Dyala PJ (1983) Osteosclerotic myeloma and peripheral neuropathy. Neurology 133: 202-210. [Crossref]
- Fukunaga H, Mutoh T, Tatewaki Y, Shimomura H, Totsune T, et al. (2017) Neuro-Myelomatosis of the Brachial Plexus – An Unusual Site of Disease Visualized by Fluorodeoxyglucose-Positron Emission Tomography/Computed Tomography (FDG-PET/CT): A Case Report. Am J Case Rep 18: 478-481. [Crossref]
2021 Copyright OAT. All rights reserv



