Brown seaweeds, especially those of the Ecklonia genus, are known to be a rich source of phlorotannins and sterols, a type of compounds which are known to have diverse biological activities. Four phlorotannins, along with a common sterol derivative, fucosterol were isolated and characterized from edible marine brown alga Ecklonia maxima. The compounds were phloroglucinol (1) (a monomer of the fucols), an eckol (2), 7-phloeckol (3), 2-phloeckol (4) and a fucosterol (5). The compounds were examined for their cytotoxic effects evaluated by use of 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) reduction assay on a limited variety of cancer cell lines. IC50 value was expressed as a sample concentration that reduced the absorbance at 560 nm by 50% of the untreated control wells. These results indicated that eckol (2) had the highest activity against HeLa cells, H157 and MCF7, making it a good candidate for consideration in the pharmaceutical and cosmeceutical industries. High-performance liquid chromatography (HPLC) was employed for the separation of the phlorotannins. NMR and mass spectrometry was used for characterization of isolated compounds. Although isolated from other species, there has not been any report on such compounds from E. maxima and their cytotoxicity effects on the selected cell lines.
Ecklonia maxima, HPLC, Phlorotannins, cell viability, Kelp
There are many brown seaweed species which grow along the coastal regions of South Africa. Ecklonia maxima kelp which flourishes on rocky reefs off the coast of the western peninsula, where the pristine, icy Benguela current sweeps up from Antarctica, has been of particular interest to the South African agricultural and pharmaceutical industries. The currents keeps the kelp fronds in constant motion, bathing them in the tumultuous, nutrient-rich ocean, resulting in their fairly rapid growth. Their emersion periods lead to both direct exposure to sunlight and potential photo-oxidative stress. As a means of protecting themselves against harmful solar radiation, particularly ultraviolet (UV) rays, they produce photo-protective compounds which comprise of several kinds of polyphenols and pigments. This seaweed possesses a variety of compounds which include carotenoids, phlorotannins and fucoidans, and which play diverse biological and ecological roles in its life cycle. Polyphenols, known as phlorotannins, are major secondary metabolites found in Ecklonia species and other brown seaweeds, where they are generally formed through the polymerization of phloroglucinol (1, 3, 5-trihydroxybenzene) [1] (Figure 1).

Figure 1. A photograph of E. maxima (Source: www.algaebase.org)
In brown algae, phlorotannins are known to provide protection against microbial infections [2,3] and the harmful effects of UV radiation [4]. During the past few decades Ecklonia species have also been reported to display broad biological properties which include antioxidant and anti-inflammatory activities [5], radical scavenging [6], anti-cancer [7], anti-allergic activity [8], anti-plasmin [9] and antibacterial activities [10], HIV-1 reverse transcriptase, protease [11], acetyl cholinesterase [12,13], as well as tyrosinase inhibitory activity [14]. They were also found to contain polysaccharides with interesting biological activities, stimulating even more studies. Among these are fucoidans (sulfated polymers of fucose) which are well known for a wide range of applications, from medicinal, nutraceutical, to cosmeceutical. In the present study, some compounds were isolated from Ecklonia maxima and characterized by spectroscopic methods. The compounds were tested for their biological activities on limited assays.
Nuclear magnetic resonance (NMR) spectra were measured on a Varian-600 instrument using CD3OD as solvent (600 MHz for 1H and 150 MHz for 13C). MS spectra were obtained from a Waters Synapt G2 spectrometer. For TLC, aluminium pre-coated Si gel 60 F254 plates were used and spots detected under ultraviolet (UV) light (254 and 365 nm) and further visualized by spraying with vanillin-H2SO4 followed by heating at ±120°C until spots were revealed. Preparative TLC was performed on a 0.5 mm thick Si gel layer coated on 20×20 cm glass plates. Column chromatography was carried out on Si gel 60 (0.040 to 0.063 mm) (230-400 Mesh ASTM, Merck) as well as Sephadex® LH-20 (Pharmacia). Phlorotannins were determined by a HPLC (Agilent 1200, system equipped with two pumps, degasser, auto-sampler, and a controller. The temperature of the column was set at 30 °C and 254 nm UV. The column consisted of a C18, (150×4.5 mm, 5 μm) with mobile phase A consisting of water/formic acid (100:0.1) and mobile phase B consisting of acetonitrile/formic acid (100:0.1). All the solvents and chemicals used in this study were of a reagent grade from commercial sources (Sigma-Aldrich, South Africa).
Collection of seaweed material
The algal material of Ecklonia maxima was harvested in January of 2012 at Kommetjie, south of Cape Town near the Cape of Good Hope, South Africa. The material was washed with running tap-water to remove sea water salts. Subsequently, the seaweed was blended into a paste that was freeze-dried, weighed and then kept at 4 °C until further use. The freeze-dried samples were subjected to both aqueous and organic solvent extraction processes.
Extraction and isolation
The phlorotannins were isolated by an extraction process involving the use of solvents of increasing polarity. Briefly, the freeze-dried material (300 g) was extracted three times with 80% MeOH and then filtered. The filtrate was evaporated at 40°C to obtain the methanol extract. Subsequently, the extract was suspended in distilled water and partitioned between hexane, dichloromethane and ethyl acetate. The n-hexane fraction was concentrated then subjected to chromatography on a silica gel column, with 250 ml volumes of CH2Cl2: ethyl acetate (10:0-0:10) as eluent, yielding 15 sub-fractions (H01-H15). Sub-fractions H03 and H04 were combined, evaporated and the residue re-crystallized from CH2Cl2 to yield fucosterol (5, 30 mg). The EtOAc fraction (5.0 g) was also subjected to chromatography on a silica gel column, eluting with 500 ml volumes of EtOAc: methanol (10:1–5:5), yielding 15 sub-fractions (E01–E15). Sub-fractions E08 and E10 (1.6 g) were combined, and further purified using sephadex LH-20 column chromatography and ethanol as eluent. Phloroglucinol (1) (25 mg) was purified from fraction E001 (50 mg) by high performance liquid chromatography (HPLC). The chromatographic flow rate was set to 0.70 ml/min and maintained at 82:18 ratio respectively. Subsequently, fraction E008 (200 mg), using the same flow rate, led to the isolation of eckol (2) (20 mg), 7-phloroeckol (3) (20 mg) and 2-phloroeckol (4) (15 mg). Finally, the purified compounds were characterized by using their 1H and 13C NMR data and 2D experiments. The structures of compounds isolated were verified by comparison with published data [6,15,16]. The chemical structures of the phlorotannins are indicated in Figure 2.
Spectral data
Compound 1: Off-white powder; 1H NMR (CD3OD, 200 MHz) δ 5.66 (3H, s, H-2, 4, 6); 13C NMR (CD3OD, 50 MHz) δ 95.5 (C-2, 4, 6), δ 160.1 (C-1, 3, 5); ES-MS m/z 125.05 [M+H]+.(Figure2).
Compound 2: light brown powder; 1H NMR (CD3OD, 600 MHz) δ 6.17 (1H, s, H-3), δ 5.97 (1H, d, J = 2.6 Hz, H-6), δ 5.96 (1H, d, J = 2.6 Hz, H-8), δ 5.95 (1H, t, J = 2.0 Hz, H-4’), δ 5.96 (2H, d, J =2.0 Hz, H-2’, 6’); 13C NMR data, see Table 1; ES-MS, m/z 371.0397 [M + H]+, (calcd, for C18H12O9, 371.0403).
Compound 3: light brown powder; 1H NMR (CD3OD, 600 MHz) δ 5.95 (1H, s, H-3), δ 5.97 (1H, d, J = 1.8 Hz, H-6), δ 5.99 (1H, d, J = 1.8 Hz, H-8), δ 5.93 (2H, s, H-3’’, 5’’), δ 6.09 (2H, J = 2.1 Hz, H-2’, 6’), δ 5.98 (1H, t, J = 1.8 Hz, H-4’); 13C NMR data, see Table 1; ES-MS m/z 495.0570 [M - H]+ (calcd for C24H16O12, 495.0564).
Compound 4: light brown powder; 1H NMR (CD3OD, 600 MHz) δ 5.97 (1H, s, H-3), δ 6.00 (1H, d, J = 2.6 Hz, H-6), δ 6.02 (1H, d, J = 2.6 Hz, H-8), δ 5.95 (2H, s, H-3’’, 5’’), δ 6.11 (2H, J = 1.8 Hz, H-2’, 6’), δ 6.01 (1H, t, J = 1.8 Hz, H-4’); 13C NMR data, see Table 1; ES-MS m/z 495.0561 [M - H]+ (calcd for C24H16O12, 495.0594).
Compound 5: white needles; 1H NMR (CDCl3, 600 MHz) δ 0.87 (1H, m, H-1ax), δ 1.12 (1H, m, H-1eq), δ 1.88 (1H, m, H-2ax), δ 2.03 (1H, m, H-2eq), δ 3.55 (1H, m, H-3), δ 2.20 (1H, m, H-4ax), δ 2.31 (1H, m, H-4eq), δ 5.37 (1H, m, H-6), δ 1.23 (1H, m, H-7ax), δ 1.54 (1H, m, H-7eq), δ 1.55 (1H, m, H-8), δ 1.47 (1H, m, H-9), δ 0.95 (1H, m, H-11ax), δ 1.53 (1H, m, H-11eq), δ 1.10 (1H, m, H-12ax), δ 1.20 (1H, m, H-12eq), δ 1.15 (1H, m, H-14), δ 1.43 (1H, m, H-15ax), δ 1.54 (1H, m, H-15eq), δ 1.00 (1H, m, H-16ax), δ 1.55 (1H, m, H-16eq), δ 1.02 (1H, m, H-17), δ 0.71 (3H, s,H-18), δ 0.99 (3H, s, H-19), δ 0.95 (1H, m, H-20), δ 1.01 (3H, d, J=6.6, H-21), δ 1.13 (2H, m, H-22), δ 2.08 (2H, m, H-23), δ 2.17 (1H, m, H-25), δ 1.00 (3H,d, J=6.9, H-26), δ 0.99 (3H,d, J=6.9, H-27), δ 5.20 (1H, q, H-28), δ 1.59 (3H, d, J=6.6, H-29). 13C NMR data, see Table 1; ES-MS data; m/z 411.3584 [M - H]+ (calcd for C29H48O1, 411.3216).
Assessment of cell viability
Cell viability was estimated using 3-(4,5-methylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay, which is a test of metabolic competence predicated upon the assessment of mitochondrial performance. It is a colorimetric assay, which is dependent on the conversion of yellow tetrazolium bromide (MTT) to its purple formazan derivative by mitochondrial succinate dehydrogenase in viable cells [17]. Since reduction of MTT can only occur in metabolically active cells, the level of activity is a measure of the viability of the cells, and hence it provides a means of assessing the viability (cell counting) and the proliferation of cells. It can also be used to determine cytotoxicity of potential medicinal agents and toxic materials, since those agents would stimulate or inhibit cell viability and growth. The cytotoxicity was evaluated by MTT reduction assay as described by Mosmann (1983) but with some modifications. The three available cell lines of choice; HeLa, H157 and MCF7, were cultured in RPM1-1640 media in T25 flask, 25 cm3 tissue culture flasks and were allowed to grow to 90% confluency in an incubator set at 37°C containing 5% CO2 atmospheric pressure for 24 hrs. The process was carried out before they were trypsinized.
The isolates were first spiked separately onto one cell line HeLa in order to determine which one will have the most significant effect on cell proliferation. This procedure was also carried out in order to assess any possible synergic effects of the isolates on the cell line. MTT results showed that the compounds 2, 3, 4 and 5 produced proliferation activity of 43.63%, 91.79%, 99.76% and 68.94% respectively at a concentration of 125 µg/ml following 24hrs incubation in relation to the control. The isolates 2 and 5 produced activities that were lower than that of the control at all the concentrations and incubation periods tested but only 2 was subjected to further analysis. Isolates 3 and 4 upon testing their absorbance, the values resulted in being higher than that of the control, and so they were not used for further proliferation studies.
The anti-proliferation activities of isolate 2 were pursued further on the three cell lines (HeLa, H157 and MCF7) which were seeded in 96-well plates at an initial density of 5 × 103 cells per well. After the cells had been incubated for 24 h at 37 °C, various concentrations of the phlorotannin were added in the growth medium with quadruplicate wells for each dilution, and the incubation was continued for 24 h. MTT solution (20 μl) at a concentration of 5 mg/ml was added and the wells were incubated again under 5% CO2 at 37 °C. After 4 h of the incubation, the plates were shaken for 5 min and the supernatants were aspirated. The culture and excess MTT were removed from each well, and 150 μl 0.04 M isopropanol was added and the plates shaken to solubilize the formazan crystals. The absorbance was measured via ELISA plate reader at the wavelength of 560 nm. Relative cell viability was evaluated in accordance with the quantity of MTT converted to the insoluble formazan salt. The data was expressed as mean percentages of the viable cells versus the respective control. The cytotoxicity was expressed as 50% cytotoxic concentration (IC50), which was the concentration of the test substances that inhibited up to 50% of the growth of various cell lines. The percentage growth proliferation was calculated using the following formula
% cell proliferation = {At /Ac} x 100
Where, At = Absorbance value of test compound; Ac = Absorbance value of control
Four phlorotannins (1, 2, 3, 4,) were isolated from ethyl acetate extract whereas fucosterol (5) was isolated from the hexane extract. The chemical structures of these compounds were unambiguously elucidated on the basis of detailed spectroscopic analysis, as well as comparison with previously published data. These structures are depicted in Figure 2. viz; phloroglucinol (1), eckol (2), 7-phloeckol (3), 2-phloeckol (4) and a fucosterol (5) as illustrated.
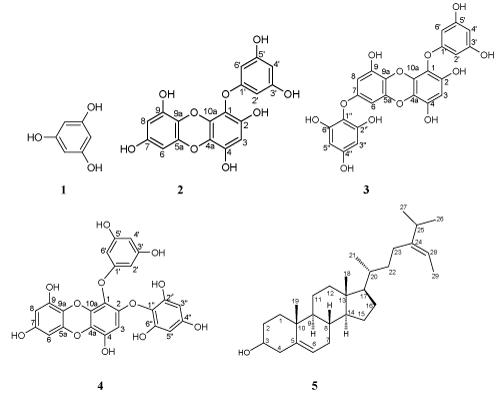
Figure 2. Chemical structures of phlorotannins (1–4) and sterol 5
Compound 1 was characterized as a symmetrical structure whose aromatic protons gave a single peak at δ 5.66 (3H, s, H-2, 4, 6). The 13C NMR spectrum displayed a peak at δ 95.5 ppm assignable to the protonated carbon atoms (C-2, 4, 6) while the signal at δ 160.1 ppm was due to the hydroxylated carbons (C-1, 3, 5). A similar compound has been reported by Rengasamy et al from the same alga [15]. In conjunction with MS results, the structure was thus identified as 1, 3, 5-trihydroxybenzene (phloroglucinol).
Compound 2 had the molecular formula C18H12O9 as determined by ES-MS, m/z 371.0397 [M + H]+, (calcd, 371.0403). 1H- and 13C- NMR data confirmed the phlorotannin nature of the structure (Table 1), as they displayed signals in the range δ 5.8-6.3 and δ C 94-163 ppm, respectively. The quaternary carbons showed characteristic peaks which could be related to the type of bonding they were involved in, i.e. either carbon to carbon, or carbon to oxygen, where the latter may in turn either be in ether-linkage or hydroxylated. Thus the signals appearing from δ 94.0-98.4 ppm could be associated with the protonated carbons (one of the peaks accounting for more than one carbon, thus totaling six), whereas the group of signals from δ 123.1-160.4 ppm was due to the non-protonated carbons, which are also oxygenated, totaling twelve. In the 1H NMR spectrum an AB2 system at δ 5.96 [(2H, J =2.0 Hz), 5.95(1H, J =2.0 Hz)], an AB system at δ 5.97 [(1H, J= 2.6 Hz), 5.96 (1H, J= 2.6 Hz)], and a singlet at δ 6.17 (1H) were observed. All 1H and 13C NMR signals were assigned with the aid of HMQC and HMBC experiments. Based on the data presented in Table 1 and comparison with the literature [18], compound 2 was identified as an eckol, characterized by the presence of a dibenzodioxin moiety. A similar compound has been reported by Rengasamy et al from the same alga and previously by others [18-22] in extracts from other brown seaweeds. Therefore, it is being reported with its systematic name as: 1-(3’,5’-dihydroxyphenoxy) dibenzo[b, e][1,4]dioxine-2,4,7,9-tetraol.
Table 1. 13C NMR data for phlorotannins (2, 3, 4) in CD3OD and sterol (5) in CDCl3
Position |
δ ppm |
|
|
Position |
δ ppm |
|
2 |
3 |
4 |
|
5 |
1 |
124.2 |
123.9 |
123.8 |
1 |
36.5 |
2 |
145.8 |
145.7 |
147.3 |
2 |
31.7 |
3 |
98.0 |
96.6 |
96.5 |
3 |
71.8 |
4 |
141.9 |
141.7 |
141.6 |
4 |
42.2 |
4a |
123.1 |
123.4 |
123.1 |
5 |
140.9 |
5a |
142.8 |
142.7 |
142.6 |
6 |
121.7 |
6 |
94.4 |
94.1 |
94.6 |
7 |
28.2 |
7 |
153.1 |
155.0 |
153.0 |
8 |
31.9 |
8 |
98.4 |
98.6 |
98.7 |
9 |
50.2 |
9 |
145.7 |
147.3 |
145.6 |
10 |
36.4 |
9a |
123.4 |
124.9 |
123.4 |
11 |
21.1 |
10a |
137.1 |
137.3 |
137.2 |
12 |
37.2 |
1′ |
160.4 |
160.6 |
160.5 |
13 |
42.4 |
2′ |
94.0 |
94.4 |
94.3 |
14 |
55.6 |
3′ |
158.8 |
159.0 |
158.8 |
15 |
24.3 |
4′ |
96.3 |
96.5 |
96.7 |
16 |
24.2 |
5′ |
158.7 |
159.0 |
158.8 |
17 |
56.7 |
6′ |
94.0 |
94.4 |
94.3 |
18 |
11.9 |
1′′ |
|
123.0 |
125.4 |
19 |
19.6 |
2′′ |
|
150.8 |
150.7 |
20 |
34.8 |
3′′ |
|
94.8 |
95.0 |
21 |
18.7 |
4′′ |
|
153.2 |
154.8 |
22 |
35.2 |
5′′ |
|
94.8 |
95.0 |
23 |
25.7 |
6′′ |
|
150.8 |
150.7 |
24 |
146.9 |
|
|
|
|
25 |
34.7 |
|
|
|
|
26 |
22.2 |
|
|
|
|
27 |
22.0 |
|
|
|
|
28 |
115.4 |
|
|
|
|
29 |
13.2 |
Compound 3 had the molecular formula C24H16O12 as determined from ES-MS data; m/z 495.0570 [M - H]+ (calcd for C24H16O12, 495.0564). The 1H NMR spectrum showed an AB2 system at δ 6.09 (2H, J = 2.1), 5.98 (1H, t), an AB system at δ 5.99 (1H, J = 1.8 Hz), 5.97 (1H, J = 1.8 Hz), and two singlets at δ 5.95 (1H) and 5.93 (2H). The signal at δ 5.93 (2H) had twice the intensity of that at δ 5.95 (1H) and twice the intensity of the doublet resonances at δ 5.97 and δ 5.99. The latter weakly coupled with each other suggesting that they are located in a meta-position relative to each other. Couplings between some of the aromatic protons were lacking indicating a certain degree of symmetrical arrangement in the molecule.
The 13C NMR spectrum indicated the presence of eight non-substituted and 16 O-bearing aromatic carbons between δ 94.4 and δ 160.6. The APT experiment indicated that the signals at δ 94.4, and δ 98.6 ppm were due to singly protonated C-atoms, with some peaks accounting for two carbon atoms each. The spectral data is very similar to that of eckol (2), except for four additional signals, suggesting that compound 3 was composed of four phloroglucinol units. The position of the extra phloroglucinol moiety was determined to be at C-7, based on the observation that the 13C NMR spectrum for compound 3 was nearly identical with that of compound 2, except for the downfield shift of the signals due to C-7 and C-9a. A similar phenomenon was reported by Okada et al [20] on elucidation of another phloroglucinol derivative, 7-phloroeckol. However, this tetramer is being reported for the first time from this alga. Consequently, it was concluded that compound 3 was 7-phloroeckol: 1-(3′,5′-dihydroxyphenoxy)-7-(2′′,4′′,6′′-trihydroxyphenoxy)-2,4,9-trihydroxy dibenzo-1,4-dioxine-2,4,9-triol, (3).
Compound 4 had the molecular formula C24H16O12 as determined from ES-MS data; m/z 495.0561 [M - H]+ (calcd for C24H16O12, 495.0564). The 1H NMR spectrum indicated an AB2 system with signals at δ 5.71 (2H, d, J = 1.8 Hz) and 5.79 (1H, d, J = 1.8 Hz). Proton “A” coupled equally to each B proton, due to their chemical and magnetic equivalence. An AB system [δ 5.77 (1H, d, J = 2.6 Hz) and 6.00 (1H, d, J = 2.6 Hz)], which displayed reflection symmetry, two singlets at δ 5.98 (1H, s), 5.92 (2H, s) and eight phenolic hydroxyl proton signals were also observed. In the 13C NMR spectrum, eight non-substituted and 16 O-bearing aromatic carbon signals were observed. The 13C NMR spectrum is very similar to that of eckol (2), except for the four extra signals, indicating that compound 4 was composed of four phloroglucinol units. The linkage position of the additional phloroglucinol moiety was determined to be at C-2 based on the fact that the 13C NMR signals for the basic skeleton in 4 were almost the same with those of 2 except for C-1, C-2 and C-3. The signals of C-1 and C-2 were observed at downfield shift compared to that of 2. An identical structure was reported by Fukuyama et al on elucidation of another phloroglucinol derivative, 2-phloroeckol [15,19,23]. The tetramer isolated is being reported for the first time from this alga. Hence, the compound was characterized as 2-phloroeckol: 1-(3′,5′-dihydroxyphenoxy)-2-(2′′,4′′,6′′-trihydroxyphenoxy)-2,4,9-trihydroxydibenzo-1,4-dioxine-4,7,9-triol, (4). Isolation of these tetramers may be predicted to have been present with regard to the season of the year. During summer, such algae undergo oxidative stress and must therefore counter excessive heat. Consequently as a way of protecting themselves against such harmful solar radiation (UV) rays, they produce photo-protective compounds such as 3 and 4 which were not reported by Rengasamy et al [18]
Compound 5 had the molecular formula C29H48O as determined from ES-MS data; m/z 411.3584 [M - H]+ (calcd for C29H48O, 411.3216). The steroidal nature of 5 was deduced from a combination of 13C, HMQC, DEPT-145 and APT NMR data, which revealed the presence of 29 carbons (6 x CH3, 10 x CH2, 9 x CH and 4 quaternary centers), four of which were olefinic (C-5, C-6, C-24, C-28). The downfield signal at δ 71.8 ppm was suggestive of a C-atom that is linked to an oxygen atom and hence could be attributed to C-3. 1H- NMR data revealed the presence of two methyl groups attached to tertiary centers: δ 0.71 (3H, s) and δ 0.99 (3H, s); four methyl groups attached to secondary centers: δ 0.99 (6H, d, J= 6.9Hz), δ 1.01 (3H, d, J= 6.6Hz) and δ 1.59 (3H, d, J= 6.6Hz), in agreement with the presence of a steroidal skeleton. The signal at δ 5.37 (1H, m) is characteristic of H-6 of sterols while the peak at δ 5.20 (1H, q) is typical of H-28 in the side chain of these structural types. The compound was thus identified as (8S,9S,10R,13R,14S,17R)-2,3,4,7,8,9,10,11,12,13,14,15,
16,17-tetradecahydro-17-(R,E)-5-isopropylhept-5-en-2-yl)-10,13-dimethyl-1H-cyclopenta-
[a]phenanthren-3-ol, a fucosterol.
The effects of the phlorotannins on viability of three cell lines (HeLa, H157 and MCF7) were measured using the MTT assay. The results were expressed as concentration of the phlorotannin required to inhibit tumor cell growth by 50% (IC50). Cytotoxicity of cisplatin, a standard, was evaluated under the same experimental conditions for comparison. The untreated cells without phlorotannins (positive control) showed 100% cell proliferation, while the viabilities of the cells pre-treated with phlorotannins were decreased with the increased concentrations of the relevant eckol; survival depends on the concentration thereof and an increase leads to decrease in survival.
The results of average absorbance and percentage proliferation were tabulated in Table 2 and graphically represented in Figure 3. It was found that the percentage cell proliferation decreased with increasing concentration up to 500 µg/ml on HeLa, H157 and MCF7 cell lines and the IC50 values less than 50 µM were considered active.
Table 2. Determination of cytotoxicity by MTT assay.
Conc.µg/ml |
HeLa cells |
H157 cells |
MCF7 cells |
|
Absorbance |
% Proliferation |
IC50 |
Absorbance |
% Proliferation |
IC50 |
Absorbance |
% Proliferation |
IC50 |
6.25 |
0.272357 |
39.70 |
˂ 50 |
0.449774 |
97.34 |
122 |
0.337917 |
48.31 |
˂ 50 |
125 |
0.233748 |
34.08 |
0.208261 |
45.07 |
0.085190 |
12.15 |
250 |
0.176023 |
25.66 |
0.089592 |
19.39 |
0.053105 |
8.01 |
500 |
0.083662 |
12.20 |
0.029397 |
6.36 |
0.048455 |
6.04 |
Cispl |
0.510261 |
70 |
0.503197 |
70 |
0.52338 |
70 |
Cont |
0.685966 |
100.0 |
0.462056 |
100.0 |
0.701071 |
100.0 |
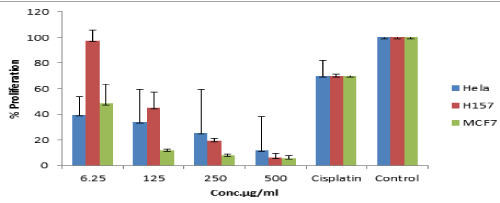
Figure 3. Activity of compound 2 on HeLa, H157 and MCF7 cell lines.
The purpose of the study was to evaluate the effectiveness of polyphenols extracted from brown algae in inhibiting carcinogenesis. Our experimental data demonstrated that topical treatment with brown algae phlorotannins inhibited the growth of HeLa, H157 and MCF7 cell lines at specific temperature. However, 6.25 µM on H157 did not inhibit the cellular growth while 125 µM gave at least 50% of inhibition. With HeLa, all the concentrations (6.25, 125, 250, 500 µM) used inhibited the cellular growth with less than 50% of inhibition. For MCF7, only 6.25 µM concentration gave at least 50% of inhibition while the other concentrations killed the cells. The H157 and MCF7 results suggested that the susceptibility of these cell lines on natural phlorotannins may be subjected to future use in cancer application. Compound 2 exhibited different activity on the selected cell lines. The selectivity was due to the sensitivity of a given cell line to the same or to tissue specific response. Based on this study, it is strongly believed that the cross killing may have occurred due to cytotoxic activity of the samples against the cell lines. Such phlorotannins are phytochemical constituents acting as major components from some brown seaweed which are responsible for the potential cytotoxic activity [24].
Marine algae have been well-known as an important source of natural bioactive secondary metabolites including phenols and polyphenols with unique linkages [25]. Ecklonia maxima, which is abundantly distributed along the South African coast, has revealed that it contains a variety of phlorotannins and a sterol derivative which have potential defensive or protective functions against cancer cells proliferation. Some further reports suggest that these phlorotannins from the algae exhibit antioxidant effects on free radicals hence offering photo-protective effect on cell damage. The viability effect against the selected cell lines may be correlated with the number and position of hydrogen-donating hydroxyl groups on the aromatic ring of the phenolic molecules. Our results indicated that compound 2 has functional hydroxyl groups that are well positioned in the dibenzodioxin moiety than other tested phlorotannins; thus, they are effectively exposed to the cells for the relevant action. Cosmeceutical and pharmaceutical industries may target this compound as a suitable natural compound lead candidate from marine biomass for cancer management.
2021 Copyright OAT. All rights reserv
The present study resulted in the isolation of four phlorotannins and a sterol from Ecklonia maxima. The phloroglucinol and the eckol have been isolated before from the same alga. However, the tetramers 3 and 4 are being reported for the first time from E. maxima. Their presence may be associated with the alga synthesis of photo protective compounds against harmful solar radiation (UV) rays. s. The isolated compounds were evaluated for their effects on metastasis on human cervical, lung and breast cancer cells. The protective effects of the compounds against the cancer cells were evaluated via the MTT assay. Among the phlorotannins, compound 2, an eckol showed prominent inhibitory activity against metastasis and effectively reduced induced cell damages. These results indicated that the compound can be used as an effective natural source of anticancer agent or as a lead compound towards drug development for the treatment of cancer.
This work was supported by a research grant from Lamicare group. We also thank the UWC Council for additional financial support and Tyson Motsoabisane of the University of Free State, South Africa for running the NMR experiments.
The authors declare that there is no conflict of interest regarding the publication of this article
- Ragan, MA, Glombitza KW (1986) Phlorotannins: brown algal poly¬phenols. Prog Phycol Res 4: 130-241.
- Waterman PG, Mole S (1994) Analysis of Phenolic Plant Metabolites. Blackwell Scientific Publications, Oxford
- Pavia H, Toth GB (2000) Inducible chemical resistance to herbivory in the brown seaweed Ascophyllum nodosum. Ecology 81: 3212-3225.
- Pavia H, Cervin G, Lindgren A, Åberg P (1997) Effects of UV-B ra¬diation and simulated herbivory on phlorotannins in the brown alga Ascophyllum nodosum. Mar Ecol Prog Sern157: 139-146.
- Kim AR, Shin TS, Lee MS, Park JY, Park KE, et al. (2009) Isolation and identification of phlorotannins from Ecklonia stolonifera with anti-oxidant and anti-inflammatory properties. J Agric Food Chem 2009, 57: 3483-3489. [Crossref]
- Li Y, Qian ZJ, Ryu B, Lee SH, Kim MM, et al. (2009) Chemical components and its antioxidant properties in vitro: an edible marine brown alga Ecklonia cava. Bioorg Med Chem 17: 1963-1973. [Crossref]
- Hashida W, Tanaka N, Kashiwada Y, Sekiya M, Ikeshiro Y, et al. (2008) Tomoeones A-H, cytotoxic phloroglucinol derivatives from Hypericum ascyron. Phytochemistry 69: 2225-2230. [Crossref]
- Li Y1, Lee SH, Le QT, Kim MM, Kim SK (2008) Anti-allergic effects of phlorotannins on histamine release via binding inhibition between IgE and Fc epsilonRI. J Agric Food Chem 56: 12073-12080. [Crossref]
- Nakayama Y, Takahashi M, Fukuyama Y, Kinzyo Z (1989) An anti-plasmin inhibitor, eckol, isolated from the brown alga Ecklonia ku¬rome Okamura. Agric Biol Chem 53: 3025-3030.
- Nagayama K, Iwamura Y, Shibata T, Hirayama I, Nakamura T (2002) Bactericidal activity of phlorotannins from the brown alga Ecklonia kurome. J Antimicrob Chemother 50: 889-893. [Crossref]
- Artan M, Li Y, Karadeniz F, Lee S, Kim M, et al. (2008) An¬ti-HIV-1 activity of phloroglucinol derivative, 6,6’-bieckol from Ecklonia cava. Bio-org Med Chem16: 7921-7926. [Crossref]
- Myung CS, Shin HC, Bao HY, Yeo SJ, Lee BH, et al. (2005) Improvement of memory by dieckol and phlorofucofuroeckol in ethanol-treated mice: possible involvement of the inhibition of acetylcholinesterase. Arch Pharm Res 28: 691-698. [Crossref]
- Yoon NY, Chung HY, Kim HR, Choi JS (2008) Acetyl- and butyryl¬cholinesterase inhibitory activities of sterols and phlorotannins from Ecklonia stolonifera. Fish Sci 74: 200-207.
- Heo SJ, Ko SC, Cha SH, Kang DH, Park HS, et al. (2009) Effects of phlorotannins isolated from Ecklonia cava on melanogenesis and their protective effect against photo- oxidative stress induced by UV-B radiation. Toxicol in vitro 23: 1123-1130. [Crossref]
- Glombitza KW, Rauwald HW, Eckhardt G (1977) Fucophlorethol, hydroxyoligophenyl ether, Fucus vesiculosus. Planta Medica 32: 33-45.
- Geiselman JA, McConnell OJ (1981) Polyphenols in brown algae Fucus vesiculosus and Ascophyllum nodosum. Chemical defences against the marine herbivorous snail, Littorina littorea. J of Chem Ecol 7: 1115-1133.
- Okada Y, Ishimaru A, Suzuki R, kuyama T (2004) A New Phloroglucinol Derivative from the Brown Alga Eisenia bicyclis: Potential for the Effective Treatment of Diabetic Complications. J Nat Prod 67: 103-105. [Crossref]
- Suzuki R, Okada Y, Okuyama T (2003) A new flavone C-glycoside from the style of Zea mays L. with glycation inhibitory activity. Chem Pharm Bull (Tokyo) 51: 1186-1188. [Crossref]
- Prema R, Sekar DS, Sekhar KB, Jeevanandham S (2012) In vitro cytotoxicity study on combined plants extracts (Cissus quadrangularis and Aegle marmelos). Euro. Journal of Exp Biol 2: 882-888.
- Torres MA, Barros MP, Campos SC, Pinto E, Rajamani S, et al. (2008) Biochemical biomarkers in algae and marine pollution: a review. Ecotoxicol Environ Saf 71: 1-15. [Crossref]
- Rengasamy RRK, Mutalib AA, Ashwell RN, Wendy AS, Johannes VS (2013) Acetylcholinesterase inhibitory activity of phlorotannins isolated from the brown alga, Ecklonia maxima (Osbeck) Papenfuss. Food Research International 54: 1250-1254.
- Lee SJ, Ko JH, Lim KT (2005) Glycine and Proline-rich Glycoprotein isolated from Solanum Nigrum activates Caspase-3 through Cytochrome-C in HT-29 cells. Oncology Reports 14: 789-796. [Crossref]
- Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J of Immunol Methods 65: 55-63. [Crossref]
- Fukuyama Y, Miura I, Kinzyo Z, Mori, Kida M, et al. (1982) Chem. Lett 739-742.
- Craigie JS, McInnes AG, Ragan MA, Walter JA (1977) Chemical constituents of the physodes of brown algae. Characterization by 1H and 13C nuclear magnetic resonance spectroscopy of oligomers of phloroglucinol from Fucus vesiculosus (L). Canadian J of Chem 55: 1575-1582.



