Abstract
Purpose: Among organ transplantation, simultaneous pancreas-kidney (SPK) transplantation is becoming increasingly common in patients with type I and II diabetes mellitus (DM). The purpose of this study is to review SPK transplantation cases and learn the lessons from the experience performed at out institution.
Methods: We retrospectively reviewed and the analyzed the medical records of 25 SPK transplantation recipients performed from January 2003 to June 2016 in Korea.
Results: 25 cases of SPK transplantation were type I DM patients. Twenty-four patients (seven males and 18 females) underwent SPK transplantation from deceased donors, and one female patient received living kidney transplantation. Enteric exocrine drainage via pancreas graft drainage was done in all cases. There were 10 acute rejection cases; all were relieved by steroid pulse therapy. Kidney graft loss occurred in four patients and two pancreatectomy graft surgeries were performed due to one graft necrosis and the other graft rejection. Insulin injection or oral hypoglycemic agents were used for three patients. With the exception of five mortality cases, 20 patients stopped insulin and oral hypoglycemic agents after transplantation. Blood glucose level was maintained within normal range without medication in 16 patients.
Conclusion: SPK transplantation is a multiple organ transplantation that requires careful treatment to avoid complications in the postoperative period. SPK transplantation can render insulin dependent type I DM patients with end stage renal disease (ESRD) free from insulin use and produce proper graft functions.
Keywords
pancreas kidney transplantation, outcome, complication
Abbreviations
ATG: Anti-Thymocyte Globulin; bid: bis in die- twice a day; DM: Diabetes Mellitus; ESRD: End Stage Renal Disease; GVHD: Graft Versus Host Disease; HLA: Human Leukocyte Antigen; INR: International Normalized Ratio; MMF: Mycophenolate Mofetil; PAK: Pancreas After Kidney; PRA: panel Reactive Antibody; PTA: Pancreas Transplant Only; SPK: Simultaneous Pancreas-Kidney; tid: ter in die- three time a day
Introduction
Diabetes mellitus (DM) underlies many micro- and macro-vascular diseases, such as retinopathy, neuropathy, nephropathy, cerebrovascular, and coronary and peripheral vascular systems. Hyperglycemia attributed to these complications can be fatal; the mortality rate is 13% after 20 years of type 1 DM (T1DM) [1]. Many people with DM require insulin injections. Exogenous insulin does compare to the physiologic insulin produced by a functional pancreas. Simultaneous pancreas-kidney (SPK) transplantation has been the ultimate treatment of DM and end-stage renal disease (ESRD) for a half-century [2].
SPK transplantation yields better graft survival than pancreas or kidney transplantation alone [3]. The benefit of SPK transplantation comes with the risks of higher morbidity and longer hospital stay [4]. Complications of pancreatic transplantation can be divided into early and late stages. Infection and vascular complications are more common in the early stage [5]. Thrombosis, leaks and infections are technical reasons that lead to graft lost. These technical failures occur more often in SPK transplantation than in pancreas after kidney (PAK) and pancreas transplant only (PTA); in SPK, thrombosis accounts for more than half of all technical failures followed by infection [6]. Efforts to reduce these technical failures may reduce the risks of SPK transplantation.
This study retrospectively reviews SPK transplantation cases conducted at single center in Korea.
Materials and methods
The present retrospective study of medical records and radiological examinations involved 25 patients underwent SPK transplantation from January 2003 to June 2016. All the cases were performed by one transplantation team. All data were recorded on a computerized database. All patients were followed-up in our center for a minimum 5 months post-transplantation.
The bench work was done with the donor iliac artery Y-graft. Implantations of both the pancreas and kidney were done intraperitoneally with the pancreas for the right iliac fossa and the kidney for the left iliac fossa. Anastomosis of the artery and vein of the graft was performed to internal or external iliac artery and external vein, with the pancreas to the right side and kidney to the left side. The gastoduodenal artery of the pancreas graft was anastomosed to the right internal iliac artery. For enteric drainage, the duodenum of the graft was anastomosed to the recipient’s jejunum by hand-sewing. The ureter of the graft was anastomosed to the recipient’s bladder using the Liche’s technique.
The patients received anti-thymocyte globulin (ATG), basiliximab, alemtuzumab, or ATG+rituximab as an induction therapy. Maintenance immunosuppression consisted of calcineurin inhibitor, mycophenolate mofetil (MMF), steroids. Calcineurin inhibitor was started twice a day for a serum trough level of 8-12 ng/mL. MMF was given at an oral dose of 540 mg twice a day. Methylprednisolone 500 mg was administrated intravenously during surgery and then rapidly tapered, with an effort made to stop completely. All recipients received infection prophylaxis consisting of antibiotics (cefotaxime 1 g tid for 5 days and ampicillin/sulbactam 1.5 g tid for 5 days) and antifungal agent (sulfamethoxazole/trimethoprim for 6 months and itraconazole 100 mg bid for 1 month). Anticoagulation therapy was routinely performed. Heparin was started intravenously after absence of postoperative bleeding was confirmed. After starting oral intake, hepatin was replaced by warfarin, which was continued for 6 months with treatment adjusted to a serum INR level 1.8-2.0. Delayed graft function of the kidney was defined as the need of hemodialysis for the first week after transplant.
After discharge, the patients were regularly followed-up at the outpatients clinic. When creatinine was elevated or hypo- or hyper- glycemia was detected, evaluation of the kidney or the pancreas was performed by Doppler sonography and a biopsy of the kidney was performed. Biopsy-proven acute cellular rejections were treated using steroid pulse therapy with methylprednisolone 500 mg for 3 days and continuously tapering steroid. A postoperative complication was defined as the need for re-operation, medication (anticoagulation or antibiotics) or intervention. Pancreas graft failure was defined as the permanent requirement for exogenous insulin after transplantation. Kidney graft failure was defined as the permanent requirement for dialysis after transplantation. Graft survival was censored for patient death with a functional graft, and patients with no record of death or graft failure were censored at the date of last follow-up. Patient survival was determined as time from first transplant to time of patient death, censoring at last follow-up where no death was reported.
All data was summarized as the median with the range, and categorical data was summarized as proportions and percentages. The Kaplan-Meier method was used to calculate graft and patient survival rates. Statistical analysis was performed using SPSS for Windows, version 22.0 software package (SPSS Inc, Chicago, IL, USA) and p values less than 0.05 was considered to be statistically significant.
Results
Twenty-five patients underwent SPK transplantation in our institution from January 2003 to June 2016. The baseline characteristics of donors and recipients are summarized in Table 1. Median age was 37 years (range, 23-58 years). It consists of seven male and eighteen female patients. All the patients were diagnosed with T1DM. Median duration following diagnosis was 19 years (range 5-28 years). Nineteen patients had hypertension. No patients had a history of coronary artery disease or cerebrovascular disease. The median duration of dialysis was 58 months (range, 3-120 months). Twenty-one patients were maintained on hemodialysis and the other four patients were on peritoneal dialysis.
Table 1. Donor and recipient baseline characteristics
Characteristic |
Value |
Recipient characteristic |
|
Age (years) |
37 (23-58) |
Male |
7 |
Female |
18 |
Pre-transplant body weight (kg) |
53.1 (40.0-87.0) |
Pre-transplant height (cm) |
161.1 (147.0-175.7) |
Pre-transplant body mass index (kg/m2) |
19.7 (15.2-30.7) |
DM |
|
Type 1 |
25 |
Duration (years) |
19 (5-28) |
Pre-transplant HbA1c (%) |
7.2 (5.8-9.6) |
DM nephropathy |
25 |
Duration of dialysis (months) |
58 (3-120) |
Dialysis methods |
|
HD |
21 |
PD |
4 |
HTN |
19 |
Cardiovascular disease |
0 |
Cerebrovascular disease |
0 |
Donor characteristic |
|
Age (years) |
29 (15-45) |
Male |
15 |
Female |
10 |
Cause of death |
|
Trauma |
9 |
Hypoxic brain damage |
4 |
Cerebrovascular accident |
12 |
Body weight (kg) |
58.7 (43.5-110.0) |
Height (cm) |
170.0 (150.4-180.0) |
Body mass index (kg/m2) |
22.0 (15.6-38.1) |
Final serum creatinine (mg/dL) |
1.02 (0.31-2.61) |
CRRT |
1 |
DM, diabetes mellitus; HD, hemodialysis; PD, peritoneal dialysis; HTN, hypertension; CRRT, continuous renal replacement therapy
The median age of the donors was 29 years (range, 15-45 years). There were 15 male and 10 female donors. The causes of death were trauma in nine, hypoxic brain damage in four, and cerebrovascular accident in 12 donors. The median body mass index of the donors was 22.0 kg/m2 (range, 15.6-38.1 kg/m2). The median level of final serum creatinine was 1.02 mg/dL (range, 0.31-2.61 mg/dL).
The operation-related characteristics are presented in Table 2. Seventeen patients were 0% panel reactive antibody (PRA) matching, two patients displayed < 50% PRA match, and four patients had were more than ≥50% PRA match. Median number of HLA mismatches was three in Class I (range, 1-3) and one in Class II (range, 0-2). Induction therapy was decided based on the risk of the patients. Eighteen patients received ATG, one patient received basiliximab, two patients received alemtuzumab, and four patients received ATG and rituximab as an induction therapy. The latter four patients were high-risk: one had received a kidney transplant, one was HLA Class I positive and B cell positive, and the remaining two were HLA Class I/II positive and DSA positive. Systemic venous drainage was performed for all pancreas transplantation cases. Pancreas exocrine drainage was performed by enteric drainage in all patients.
Table 2. Operation-related characteristics
Operation-related characteristics |
Value |
PRA |
|
0% |
17 |
< 50% |
2 |
≥ 50% |
4 |
HLA mismatch |
|
Class I |
3 (1-3) |
Class II |
1 (0-2) |
Induction therapy |
|
ATG |
18 |
Baxiliximab |
1 |
Alemtuzumab |
2 |
ATG+Rituximab |
4 |
Enteric drainage |
25 |
Systemic venous drainage |
25 |
ATG, anti-thymocyte globulin; HLA, human leukocyte antigen; PRA, panel reactive antibody
The outcomes of the transplantation are summarized in Table 3. Median follow-up was 43 months (range, 1-165 months). Delayed graft function of the kidney occurred in four patients (16%) and biopsy-proved acute rejection occurred in 10 patients (40%). The median serum creatinine levels were 1.07 mg/dL (range, 0.83-2.58 mg/dL) at 1 year, 1.16 mg/dL (range, 0.77-1.31 mg/dL) at 3 years, 1.13 mg/dL (range, 0.73-1.36 mg/dL) at 5 years and 1.19 mg/dL (range, 0.74-1.35 mg/dL) at 10 years. The median hemoglobin A1C was 5.4% (range, 4.3-10.5%) at 1 year, 5.0% (range, 4.6-9.8%) at 3 years, 5.6% (range, 5.1-10.1%) at 5 years and 5.8% (range, 5.1-6.0%) at 10 years. The patient survival rates were 88.0% at 1 and 3 years, 81.2% at 5 years and 71.1% at 10 years (Figure 1). The graft survival rates for the pancreas were 75.0% at 1 and 3 years, 68.7% at 5 years and 57.3% at 10 years (Figure 2A). Two pancreas graftectomies were performed because of graft necrosis and uncontrolled infection. Another patient restarted exogenous insulin therapy and two patients received oral DM medication. The kidney graft survival rates were 84.0% at 1 year, 78.0% at 3 years, 71.5% at 5 years and 61.3% at 10 years (Figure 2B).
Table 3. Outcomes of transplantation
Outcomes |
Value |
Postoperative complications |
|
Bleeding |
8 |
Vascular thrombosis |
4 |
Intraabdominal sepsis or infection |
6 |
Urinary tract obstruction |
2 |
Wound problem |
3 |
Hospital day (day) |
43 (20-240) |
Mortality at 30 days |
0 |
Follow-up (month) |
44 (1-165) |
Delayed graft function (%) |
4 (16) |
Biopsy-proved acute rejection |
10 |
Serum creatinine level (mg/dL) |
|
1 year |
1.07 (0.83-2.58) |
3 years |
1.16 (0.77-1.31) |
5 years |
1.13 (0.73-1.36) |
10 years |
1.19 (0.74-1.35) |
HbA1c (%) |
|
1 year |
5.4 (4.3-10.5) |
3 years |
5.0 (4.6-9.8) |
5 years |
5.6 (5.1-10.1) |
10 years |
5.8 (5.1-6.0) |
Graft survival rates (%) |
|
Kidney |
|
1/3/5/10 years |
84.0/78.0/71.5/61.3 |
Pancreas |
|
1/3/5/10 years |
75.0/75.0/68.7/57.3 |
Patient 5 year survival rates (%) |
81.2 |
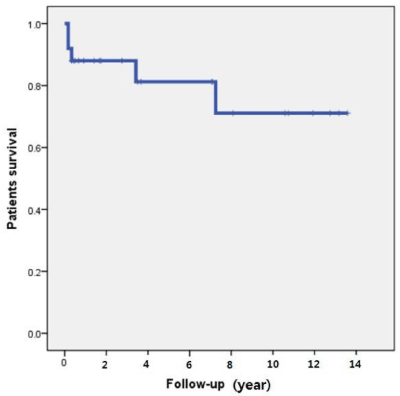
Figure 1. Patient survival rate.
2021 Copyright OAT. All rights reserv
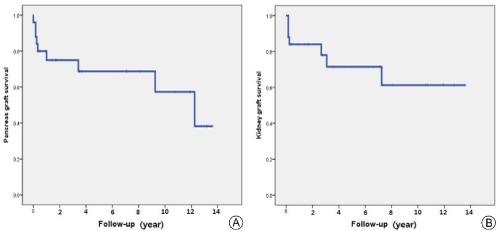
Figure 2. Graft Survival rate. (A) Pancreas graft survival rate, (B) Kidney graft survival rate.
Discussion
SPK transplantation is the ultimate cure for insulin-dependent DM and ESRD. SPK transplantation outcomes and survival rates are better than pancreas or kidney transplantation alone [7]. This study shows long-term results from our initial to the present time at our center, although the number of patients is small. The graft survival rate of kidney and pancreas is similar to that of other institution [8].
Five patients died. One had received a graftectomy after 15 days due to graft necrosis. Another patient had been diagnosed as rejection due to deterioration of graft function; the graft was removed 3 months after transplantation to control an infection. Four of the other patients received experienced insulin injections or oral diabetes medication due to graft failure. The remaining 16 patients were successfully weaned off insulin.
Of the five patients who died, one died of hypoxic brain damage due to hypoglycemia. The other four deaths were due to infection. One patient underwent emergent surgery for acute cholecystitis and empyema 15 days after SPK transplantation and died 2 months after transplantation of an uncontrolled Candida peritonitis infection. One patient died of intraabdominal bleeding and sepsis due to intestinal perforation 4 months after the SPK transplantation. One patient died within 41 months of an intraperitoneal infection due to duodenal perforation at 39 months after transplantation. One patient died of peritoneal infection due to small bowel perforation during treatment with graft versus host disease (GVHD) 1.5 months after SPK transplantation. In addition to these patients, one patient had undergone a graftectomy for uncontrolled infection due to graft failure as well as rejection. Transplant patients are known to be at risk of infection because of the use of immunosuppressants. In addition, intraabdominal infections after SPK transplantation are directly related to death; this risk is more pronounced than flowing solid organ transplantation.
The early postoperative period is still considered a high-risk period for a relaparotomy [9]. This suggests that surgical complications are associated with strong non-immunologic complications after transplantation in the early postoperative period [10]. Anticoagulation therapy after SPK transplantation is also involved. Anticoagulation therapy begins immediately without any evidence of bleeding after transplantation. Therefore, the risk of recurrent bleeding is high. Even if the re-bleeding is stopped, intraabdominal hematoma can cause the infection and the complication of relaparotomy cannot be overlooked if reoperation is done to remove hematoma or to prevent bleeding. However, restriction of the coagulation to prevent bleeding can cause intravenous thrombosis resulting in graft loss. Fine control between bleeding and thrombosis after SPK transplantation is very important.
Duodenal leakage is also a potential complication after pancreas transplantation. Graftectomy should be considered as a life-saving measure when duodenal segment leakage with abdominal sepsis occurs or upon prolonged morbidity with non-healing leakage [11]. Duodenal leakage has not occurred in any cases at our center, and it seems to be influenced by the supply of blood flow to the inferior pancreaticoduodenal arcade of the graft by connecting the gastroduodenal artery with internal iliac artery.
It is not easy to find a suitable deceased donor because the SPK transplantation basically requires a healthy donor without underlying disease under the age of 45 years. In Korea, there are a few large transplant centers, but there are not many other SPK transplantation experienced teams. In addition, SPK transplantation is difficult to perform in small institution because it requires a collaborative teamwork with a diverse team including transplant physicians, radiologist, endocrinologists, nutritionists and pharmacists. Although the results of the present study are not inferior to those of large overseas institutions, the results could be further improved through surgical complication improvement, infection control and accurate anticoagulation therapy system.
Author contributions
Ji Soo Lee contributed to the design of the study and drafting the manuscript. Jae Berm Park contributed to the design of the study and revising the manuscript. Hyo Jun Park contributed to data acquisition. Kyeong Sik Kim contributed to data acquisition. Chan Woo Cho contributed to data analysis. Kyo Won Lee contributed to data analysis. Sung Joo Kim contributed to the design of the study and revising the manuscript.
Disclosures statement
The authors have no conflict of interest to declare.
Funding
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript.
References
- Stadler M, Auinger M, Anderwald C, Kästenbauer T, Kramar R, et al. (2006) Long-term mortality and incidence of renal dialysis and transplantation in type 1 diabetes mellitus. J Clin Endocrinol Metab 91: 3814-3820. [Crossref]
- Kleespies A, Mikhailov M, Khalil PN, Preissler G, Rentsch M, et al. (2011) Enteric conversion after pancreatic transplantation: resolution of symptoms and long-term results. Clin Transplant 25: 549-560. [Crossref]
- Reddy KS, Stablein D, Taranto S, Stratta RJ, Johnston TD, et al. (2003) Long-term survival following simultaneous kidney-pancreas transplantation versus kidney transplantation alone in patients with type 1 diabetes mellitus and renal failure. Am J Kidney Dis 41: 464-470. [Crossref]
- Lee CM, Scandling JD, Krieger NR, Dafoe DC, Alfrey EJ (1997) Outcomes in diabetic patients after simultaneous pancreas-kidney versus kidney alone transplantation. Transplantation 64: 1288-1294. [Crossref]
- Franca M, Certo M, Martins L, Varzim P, Teixeira M, et al. (2010) Imaging of pancreas transplantation and its complications. Insights Imaging 1: 329-338. [Crossref]
- Humar A, Ramcharan T, Kandaswamy R, Gruessner RW, Gruessner AC, et al. (2004) Technical failures after pancreas transplants: why grafts fail and the risk factors--a multivariate analysis. Transplantation 78: 1188-1192. [Crossref]
- Stratta RJ, Farney AC, Orlando G, Farooq U, Al-Shraideh Y, et al. (2014) Similar results with solitary pancreas transplantation compared with simultaneous pancreas-kidney transplantation in the new millennium. Transplant Proc 46: 1924-1927. [Crossref]
- Sampaio MS, Kuo HT, Bunnapradist S (2011) Outcomes of simultaneous pancreas-kidney transplantation in type 2 diabetic recipients. Clin J Am Soc Nephrol 6: 1198-1206. [Crossref]
- Page M, Rimmele T, Ber CE, Christin F, Badet L, et al. (2012) Early relaparotomy after simultaneous pancreas-kidney transplantation. Transplantation 94: 159-164. [Crossref]
- Banga N, Hadjianastassiou VG, Mamode N, Calder F, Olsburgh J, et al. (2012) Outcome of surgical complications following simultaneous pancreas-kidney transplantation. Nephrol Dial Transplant 27: 1658-1663. [Crossref]
- Singh RP, Vrakas G, Hayek S, Hayek S, Anam S, et al. (2013) Clinically significant peripancreatic fluid collections after simultaneous pancreas-kidney transplantation. Transplantation 95: 1263-1269. [Crossref]


