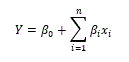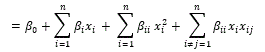Bacopa monniera (Brahmi) is widely used as traditional medicine to treat cognitive disorders. It contains several bioactive compounds which are bitter in taste when consumed. Therefore, encapsulation was carried-out to prepare Bacopa monniera extract (BME) nanoparticles. To optimize the condition for nano-encapsulation of extract, central composite rotatable design (CCRD) of response surface methodology (RSM) was implemented. Chitosan (%), TPP (%) and BME concentration (mg) levels were taken as variables and size and zeta potential as responses. Regression analysis for two responses such as size and zeta potential were conducted by fitting the quadratic model. The optimized conditions were (0.1% chitosan, 0.15 % TPP and 15 mg/ml of BME) and showed a high zeta potential value of about +48.5mV and size as 220 ± 12nm. HPLC, total polyphenol contents, antioxidant assays (DPPH, ABTS) were carried out to ensure the efficiency of the encapsulation and it was found to be 52.0 %.
Bacopa monniera, RSM, nanoencapsulation, antioxidant.
A variety of functional foods and dietary supplements have been introduced to the market over the past few years, with the promising prospects for the improvement of the public health. However the concern of the research community is that the bioactive compounds may not be fully absorbed into the body, because they had been degraded before reaching the target organ. The efficacy of bioactive compounds may be reduced due to their instability caused by harsh acidic condition, enzymatic reactions and low permeability in the gastrointestinal tract. Therefore, the delivery and release of bioactive cores from ingestion to digestion have been recognized as the most important criteria in improving their absorption [1].
Bacopa monniera is a member of Scrophulariaceae, hold great promise for the improvement of cognitive and endurance enhancing function. It is an important constituent of the ayurvedic medica and classified into medhyarasayana, a drug known to improve memory and intellect [2]. Extensive investigations indicated that the cognition facilitating effect of B. monniera was due to the presence of active saponins, bacoside A [3]. These active principles, apart from facilitating learning and memory in normal rats, it has several other health benefits such as antioxidant [4-6]. anti-stress [7], vasodilator [8], anti-inflammatory [9], anxiolytic [10]. In our earlier studies we have demonstrated the anti-fatigue property [11], neuroprotective property of Bacopa monniera against various neurotoxicants such as hydrogen peroxide, scopolamine, sodium nitroprusside and crackers smoke [12-14].
Nanotechnology applications were initially implemented to prevent cancer, decrease toxicity, increase stability and bioavailability and promote selective tumor uptake[15]. More recently, these approaches are extended in cancer prevention [15] with dietary phytochemicals [16]. As a result, a new area of investigation, nano-chemoprevention , was born which holds promise to enhance the efficacy of bioactive food compounds through nanoencapsulation. In fact, recent studies showed that nanoparticles of epigallocatechin-3-gallate (EGCG) from green tea, curcumin from turmeric, and resveratrol from table grapes are more efficacious than their free counterparts [17-19].
Response Surface Methodology (RSM) explores the relationships between several explanatory variables and one or more response variables. RSM is a collection of mathematical and statistical techniques useful for the modeling and analysis of problems in which a response of interest is influenced by several variables and the objective is to optimize this response [20]. Originally, RSM was developed to model experimental responses, and then migrated into the modelling of numerical experiments [21]. The application of RSM to design optimization is aimed at reducing the cost of expensive analysis methods and their associated numerical noise. Statistical design tools such as RSM helps quite effectively in optimizing the independent variable levels in product and process parameters based on statistical and mathematical techniques. The RSM technique gives the effect of an individual parameter as well as interactive effect of the parameters [22]. This statistical tool has been employed for the standardization and optimizing processing variables for different products like mutton manchurian, designer chicken shreds [23,24] food-processing operations [25] and carrot dehydration process [26].
Based on the previous interesting results of our studies on bacoside rich extract, present study was under taken to standardize and optimize of the conditions for nanoencapsulation of bacoside by RSM technique to efficiently encapsulate and mask the bitterness and bioavailability of the extract.
Chemicals
Chitosan, Ascorbic acid, 1,1diphenyl-2picrylhydrazyl (DPPH), 2,2’-azinobis (3-ethylbenzothiazoline-6-sulphonic acid) (ABTS), Tripolyphosphate, were purchased from Sigma-Aldrich Chemicals, (St. Louis, MO, USA). Acetic acid (HPLC grade), methanol (HPLC grade), ethanol, phosphoric acid, gallic acid, acetonitrile, sodium hydroxide, tetrabutyl hydroxyl quinone, folin ciocalteau’s reagent, sodium carbonate, potassium persulphate were purchased from Qualigens India ltd, Mumbai, India. Bacoside standard was purchased from M/s Natural Remedies Pvt, Ltd, Bengaluru, India.
Experimental design
The central composite rotatable design (CCRD) of RSM was used for designing the experimental combinations for the optimization of conditions for nano-encapsulation of bacoside using software State-Ease (Design Expert version 6.0.10). Chitosan (%), TPP (%) and BME concentration (mg) levels were taken as variables and size and zeta potential as responses. The number of design points was obtained with the help of statistical software depending on the number of independent variables. Design consisted of seven factorial points, six central points and seven axial points leading to 21 sets of experiments [27]. Design of experiments for the optimization of conditions for nano-encapsulation of bacoside was shown in Table 1. The results for the central composite designs were used to fit second-order polynomial equation. The regression analysis of the responses namely size and zeta potential were carried out by fitting with suitable models represented by (1) & (2). All variables of the polynomial regression at a significance level of p < 0.05 were included in the model, and the coefficient of determination (R2) was generated in order to assess the accuracy of the model. The response surfaces were generated from the equation of the second order polynomial, using the values of each independent variable to the maximum quadratic response [28].
First order Linear Equation (1)
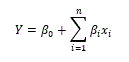
Second-order polynomial Equation (2)
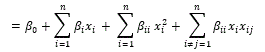
where, 0 was the value of the fitted response at the canter point of the design; i, ii and ij were the linear, quadratic and cross product (interaction effect) regression terms respectively and n denoted the number of independent variables.
Preparation of Nanoparticles
Appropriate percent amount of chitosan was dissolved in 1% acetic acid and kept in magnetic stirrer for about 45 minutes to dissolve completely. Different percent concentrations of tripolyphosphate were dissolved in milli-Q water completely to get a clear solution. Bacopa monniera extract (BME) of different concentrations were prepared by dissolving Bacopa monniera extract (lyophilized powder) in 10 ml of milli-Q water. Extract was filtered through 0.22 µm syringe filter and used for further preparation. A dry clean autoclaved beaker of 100 ml capacity was taken and 5 ml of chitosan solution was added. BME solution of about 0.75 ml is added to the above and kept in a magnetic stirrer for 15 min at 1000 rpm at 37º C. TPP of 1.5 ml is added at the rate of 200 µl/min slowly and steadily to maintain the uniformity of charge and potential. The above reaction mixture was continued to be in magnetic stirrer for about 45 minutes, at 1000 rpm, and 37º C was maintained (Table 1).
After the completion of the stirring 1 ml the reaction mixture was transferred to different 2 ml vials and subjected to centrifugation at 13,000 rpm for 13 min at 4º C. The supernatant was collected to a fresh tube to carry out encapsulation efficiency assays and the pellet was redissolved in 1 ml of milli-Q water and subjected to centrifugation at 13,000 rpm for 13 minutes at 4º C and the protocol was repeated thrice. Redissoved pellet was mixed well with micro pipette and was sonicated at sterile conditions for about 30 minutes and was further subjected to characterization of size and potential.
Characterization of Bacoside by HPLC
The amount of bacoside present in the crude and supernatant samples were quantified by using a Jasco LC-NetII/ADC HPLC system equipped with a HiQSil C18HS (150 × 4.6 mm, 5 µm partical size) column, photodiode array detector (PDA) and PU1580 pump (Jasco, USA), and a rheodyne injector with 20 µl loop. The mobile phase consisted of 0.2% phosphoric acid and acetonitrile (65:35 v/v). The pH of the mobile phase was adjusted to 3.0 with 5M NaOH. The flow rate and total run time were 1.0 mL/min and 60 min respectively. A 20 µl of standard, crude extract sample and supernatant sample was injected and analyzed. All peaks were integrated at the wavelength of 205 nm. They were initially assigned by comparing retention times with standard and compared with characteristic spectra obtained from the PDA [29]. The efficiency of encapsulation of BME was compared with standard.
DPPH Radical Scavenging Activity
The antioxidant activity of bacoside rich extract was checked on the basis of 1, 1 diphenyl-2picrylhydrazyl (DPPH) free radical scavenging activity. DPPH assay was performed as per the method described by Eberhardt et al. [30]. DPPH (500µl, 0.5 mM in methanol) solution was mixed with different amounts of sample and volume was made to 3.5 ml with methanol. The mixture was incubated in dark for 45 min at room temperature. Absorbance was recorded at 550 nm in a spectrophotometer. Tetrabutyl hydroxyl quinone was used as a standard compound. A positive control was prepared by mixing 3 ml methanol without DPPH solution to eliminate the absorbance of crude extracts. Methanol was used as blank. The DPPH radical scavenging activity percentage was calculated by using the formula as given below:
DPPH radical scavenging activity (%) = {(Ac –As)/Ac} ×100
Where Ac is the absorbance of the positive control solution and As is the absorbance of test solution. IC50 value, the concentration of sample or extract required to scavenge 50% of the DPPH free radicals in the mixture, was calculated using a linear regression equation derived from the graph of % DPPH scavenging activity and sample concentration.
Total polyphenol content
The TPC was determined according to the Folin Ciocalteau’s method [31]. The reaction mixture was composed of 0.1 ml of extract, 2.9 ml of milli-Q water, 0.5 ml of FC reagent and 2 ml of 7% sodium carbonate. The reaction mixture was incubated at room temperature for 90mins. The absorbance was measured at 735 nm. The TPC was determined as Gallic acid equivalent of extracts. The phospho-molybdate in FC reacts under alkaline condition to give a blue color that is read at 735 nm.
ABTS assay: ABTS assay was carried out as per the method of Cai et al. (2004) [32]. The ABTS radical cation (ABTS*) solution was prepared by mixing 7 mm ABTS and 2.45 mm potassium persulphate and incubated in the dark at room temperature for 16 hr. The ABTS* solution was then diluted with 80% (v/v) ethanol to obtain an absorbance of 0.700±0.005 at 734 nm. ABTS* solution (3.9 ml) was added to 0.1 ml of the test sample (pre-diluted at a ratio of 1:50) and mixed vigorously. The reaction mixture was allowed to stand at 23º C for 6 min and the absorbance was recorded at 734nm immediately. A standard curve was obtained by using ascorbic acid in 80% ethanol. The % ABTS which was scavenged (%ABTSsc) was calculated using the formula:
(%ABTSsc= (Acon-Asample) × 100/ Acon
Where Acon is the absorbance of the control and Asample is the absorbance of the sample read at 734 nm.
Preparation of FITC labeled nanoparticles
Five milligrams of FITC was weighed and dissolved in 1 ml of 100% ethanol and 24 ml of milli Q water was added to makeup and was stored in dark at 4o C. Nanoparticles were prepared using the FITC dye into Bacopa extract with five different volumes of dye as follows: (a) 375 µl of 2X BME + 50 µl of FITC dye + 325 µl of water= 750, (b) 375 µl of 2X BME +100 µl of FITC dye + 275 µl of water= 750, (c) 375 µl of 2X BME +150 µl of FITC dye + 225 µl of water= 750, (d) 375 µl of 2X of BME + 200 µl of FITC dye + 175 µl of water= 750 and (e) 375 µl of 2X of BME +250 µl of FITC dye + 125 µl of water= 750. All the 5 different conditions were done to check the optimum concentration of FITC labeled nanoparticles would fluoresce under microscope.
Internalization of FITC labeled nanoparticles into the SKNSH cells
The SK-N-SH human neuroblastoma cell line was used in this study was supplied by the NCCS, Pune, India. The cells were grown in minimal essential medium containing 10% fetal bovine serum with penicillin (100 u/ml) and streptomycin (100 mg/ml) (Sigma, St. Louis, MO, USA) in a humid atmosphere of 5% CO2 and 95% air at 37o C for all the experiments with 80% confluence and more than 95% viability was considered as optimum. All the experiments were performed in serum free media. Cells were grown on cover slips and 50 µl of FITC labeled particles were added to the cells and incubated for 24 hr. All the above steps were carried out in a dark environment. Individual slide was observed under fluorescent microscopes for its internalization into the cells [33].
Statistical analysis
Response surfaces were generated using the Design Expert version 6.0.10 software (Stat Ease Inc., Minneapolis, MN). The data obtained were subjected to analysis of variance (ANOVA) and Duncan’s multiple range test to evaluate the statistical significance of the treatments and significance was established at p<0.05.
Consumption of raw Bacoside extract is not that efficiently accepted in day today life because of bitter in taste. Optimization of conditions for nano-encapsulation of bacoside with addition of chitosan and TPP were carried out in order to reduce the bitterness of the extract. The CCRD of RSM was used for designing the experimental combinations for the optimization of conditions for nano-encapsulation of bacosides. Parameters such as Chitosan (%), TPP (%) and BME extract concentration (mg) levels were taken as variables and size and zeta potential as response. Properties of chitosan such as biocompatibility, non-toxicity and biodegradability, made it a favorable candidate for wall material in food encapsulation [33,34]. In addition chitosan has a mucoadhesive character, which is an advantage for extensive use in controlled delivery application. Hence, as a component of wall materials in a controlled release formula, chitosan has the potential to prolong residence in the delivery of the core material. TPP being the negatively charged molecule plays vital role in holding of two chitosan molecule which is positively charged.
Fitting the Model
Central composite design results were used to fit the second order polynomial equation. Regression analysis of all the two responses such as size and zeta potential were conducted by fitting the quadratic model. Analysis of variance was calculated and model statistics for all the responses were shown in Table 2. All the responses showed highly significant and fitted with quadratic and linear models. The p-value showed as p<F which should be always less than 0.05 for model to significant. The response surface plots for these two models can be plotted as a function of two variables by keeping other variables at optimum or constant levels. The effect of variations in the levels of variables (Chitosan, TPP and BME concentration) in the present design on two responses (size and zeta potential) has been depicted in 3D response plots in Figure 1. Multiple regression equations generated for all two responses are represented as follows in actual factors:
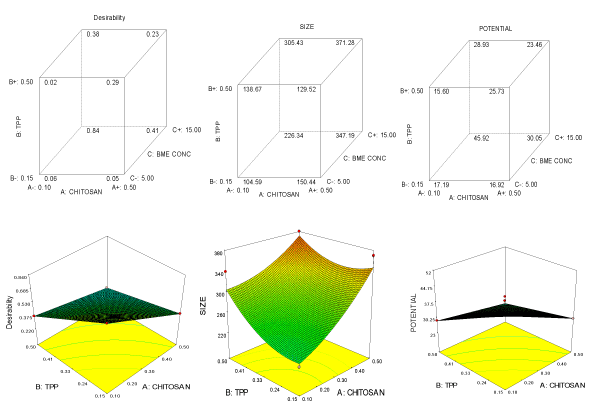
Figure 1. Cube graph of variables Chitosan, TPP and BME for (I) Desirability, (II) Size (III) Potential; 3 D plot depicting effect of independent variables Chitosan, TPP and BME for (I) Desirability, (II) Size (III) Potential.
Final equation in terms of actual factors
Size = +241.34 -153.76 *chitosan-434.44 *TPP =-26.99 *BMEconc-392.85 *chitosan *TPP18.750 *chitosan *BMEconc+12.85 *TPP *BMEconc+389.27 *chitosan2+779.73 *TPP2+1.76 *BME conc2
Potential = -0.55+7.65 *chitosan+10.02 *TPP+3.92 *BME conc +74.28* chitosan *TPP-3.90 *chitosan *BME conc-4.40 *TPP *BME conc
Responses were optimized using Design Expert version-6 software. Optimization of the independent variable levels (Chitosan, TPP and BME) was achieved based on the maximization of the responses (size and potential). From this design best among the suitable desirability was taken as optimized ingredients level. Nanoparticle was developed with optimized levels of variables and responses were analyzed and verified with the predicted values and these are shown in Table 2. It showed that predicted and actual the values were almost similar. Hence, the above levels of ingredients were recommended for the optimization of the product.
HPLC
Samples with the charge of ± 30 mV supernatants were analyzed by HPLC for its residual amounts of bacoside extract and were integrated with the crude and supernatant samples (Figure 2). Chromatogram shows that the Bacopa monniera extract was encapsulated in the form of nanoparticles.
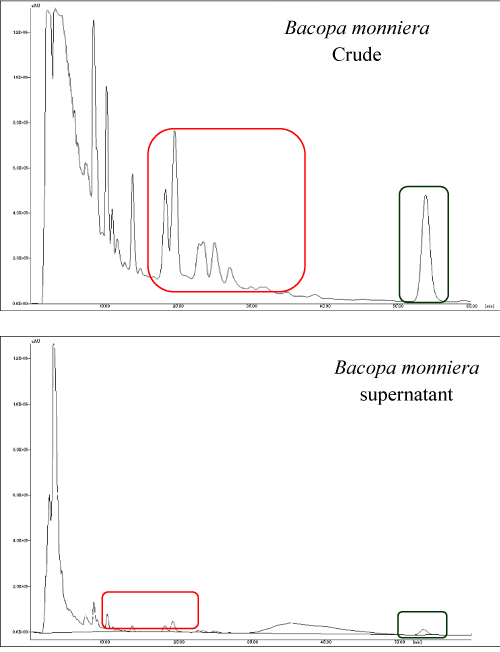
Figure 2. Quantification of bacoside encapsulation by HPLC analysis revealed different peaks of the crude (Bacopa monniera) and the supernatant left over after the encapsulation of BME. Encapsulation of active compounds shows the reduced size of chromatogram peaks.
Total Polyphenols Assay
Polyphenolics compounds have been known to combat free radical scavenging, various diseases and dietary use of the same has been advocated. In the present investigation the phenolic content of brahmi that was estimated in the supernatants of nanoencapsulated mixture in terms of gallic acid equivalent. There are other reports which suggest differential phenolic content in the test plant, which might be due to topographical and environmental effect which leads to variation in metabolite content [35,36]. According to TPC assay, the encapsulation efficiency was maximum for the sample prepared with the parameters of 0.1% chitosan, 0.15% TPP and 15 mg/ml, around 52.00% when compared to other test samples (Figure 3).
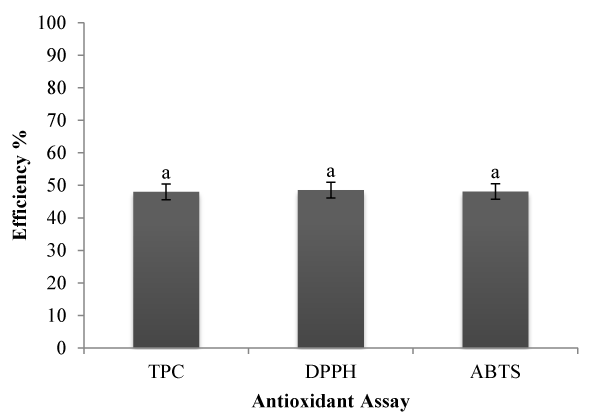
Figure 3. Total polyphenols and antioxidant asaays of the optimised nanoparticles leftover supernatant sample. Mean values of triplicates; SDs are denoted as bars. The values superscripted with different letters varies significantly (p<0.05).
DPPH Assay
The antioxidant activity of different extracts was calculated as their capacity to scavenge free radicals of DPPH, which has been widely used to evaluate the antioxidant activity of natural products from plant and microbial sources. Although radical scavenging activity should not be considered as being synonyms with antioxidant activity, it is a fact that almost all the powerful natural antioxidants, such as tocopherol, carnosol and ascorbic acid are also strong scavenger of DPPH radical. Thus, good activity in this test is also an indication of the presence of possible antioxidants. BHA was used as a standard. With the correlation of IC50 and regression equations derived from standards and the test samples, the efficiency of the BME encapsulation was calculated. Encapsulation efficiency of the assay was shown in the Table 3, as 0.1% chitosan, 0.15% TPP and 15 mg/ml showed maximum encapsulation of 52.00 % when compared to other test samples (Figure 3).
ABTS Assay
2021 Copyright OAT. All rights reserv
The scavenging activity of the ABTS with respect to the supernatant of the nano-encapsulated Bacopa monniera extract, which gives the highest yield of antioxidant with desired antioxidant activities were selected as test sample. With the correlation of the standards, the test sample was analyzed for the encapsulation efficiency. The optimized conditions from the RSM technique for (0.1% chitosan, 0.15% TPP and 15 mg/ml) revealed a maximum encapsulation efficiency of 51.90% when compared to other test samples (Figure 3).
Internalization of FITC labeled nanoparticles
SK-N-SH is a neuroblastoma cell line that displays epithelial morphology and grows in adherent culture. These cells were treated with different concentrations of FITC labeled nanoparticles and incubated for 24 hr. After incubation for 24 hr, these cells were observed under fluorescent microscope to determine the internalization of nanoparticles into the cells. Various volumes of FITC solution was added into to the solution along with the brahmi extract and evaluated that the required amount was found to be 250 µl of FITC of 200µg/ml concentration was the minimum concentration to be required to be visualized under the fluorescence microscope (Figure 4).
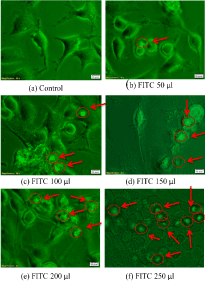
Figure 4. Internalization of nanoaparticles into the SK-N-SH cells was observed under the fluorescence microscope at 40x magnification. Nanoparticles were circled and indicated with red arrows.
The present study reveals that the Chitosan-TPP based nanoparticles of bacoside extract was prepared using the response surface methodology. The results showed that the concentration of chitosan (0.1%), tripolyphosphate (0,15%), bacoside extract (BME) (15 mg/ml) has shown to be optimum concentration to encapsulate bacosides in nanosize. Since, it maintained optimum size (226.3 nm) and zeta potential (48.5 mv). Overall, our study suggests that the model obtained in the present study can be applied for large scale production of nanoparticles for further use in pharma/food industries. This may ease the fortification/supplementation of bioactive compounds in various food formulations designed to combat performance enhancement and mitigate the cognitive impairments, health problems by their biochemical and physiological processes. Further studies are warranted to test its efficiency in vitro and in vivo models for its toxicity and bioavailability of the nanoparticles.
- Bacopa monniera Nanoparticles were prepared using the central composite rotatable design (CCRD) of response surface methodology (RSM).
- The optimized conditions were (0.1% chitosan, 0.15 % TPP and 15 mg/ml of BME) and showed a high zeta potential value of about + 48.5 mV and size as ± 226.3 nm.
- Analytical results of HPLC, total polyphenol contents, antioxidant assays (DPPH, ABTS) were carried out to ensure the efficiency of the encapsulation and it was found to be 52.0 %.
The authors have declared that there is no conflict of interest.
This work was financially supported by DRDO, India under the project of DFR-297. The authors are also thankful to the Director, Defence Food Research Laboratory, India, for their constant encouragement and support to carry out the research work.
- Agnihotri SA, Mallikarjuna NN, Aminabhavi TM (2004) Recent advances on chitosan- based micro and nanoparticles in drug delivery. Controlled Release 100: 5-28.
- Satyavati GV, Raina MK, Sharma M (1976) Medicinal Plants of India, ICRM: New Delhi, pp: 118-122.
- Singh HK, Dhawan BN (1992) Drugs Affecting Learning and Memory, Wiley Eastern: New Delhi, pp:189-207.
- Bhattacharya SK, Bhattacharya A, Kumar A, Ghosal S (2000) Antioxidant activity of Bacopa monniera in rat frontal cortex, striatum, and hippocampus. Phytother Res 14: 174-179.
- Russo A, Borrelli F, Campisi A (2003a) Nitric oxide related toxicity in cultured astrocytes: effects of Bacopa monniera. Life Sci 73: 1517-1526.
- Russo A, Izzo A, Borrelli F (2003b) Free radical scavenging capacity and protective effect of Bacopa monniera (L) on DNA damage. Phytother Res 17: 870-875.
- Chowdhuri DK, Parmar D, Kakkar P, Shukla R, Seth PK, et al. (2002) Antistress effects of bacosides of Bacopa monnieri: modulation of Hsp70 expression, superoxide dismutase and cytochrome P450 activity in rat brain. Phytother Res 16: 639-645. [Crossref]
- Channa S, Dar A, Yaqoob M, Anjum S, Sultani Z (2003) Broncho-vasodilatory activity of fractions and pure constituents isolated from Bacopa monniera. J Ethnopharmacol 86: 27-35.
- Channa S, Dar A, Anjum S, Yaqoob M, Atta UR (2006) Anti-inflammatory activity of Bacopa monniera in rodents. J Ethnopharmacol 104: 286-289
- Ernst E (2006) Herbal remedies for anxiety - a systematic review of controlled clinical trials Phytomedicine. Int J Phytother Phytopharmacol 13: 205-208.
- Anand T, Phani Kumar G, Pandareesh MD, Swamy MSL, Khanum F, et al. (2012) Effect of bacoside extract from Bacopa monniera on physical fatigue induced by forced swimming. Photother Res 26: 587-593.
- Pandareesh MD, Anand T (2013) Neuromodulatory propensity of Bacopa monniera against scopolamine-induced cytotoxicity in PC12 cells via down-regulation of AChE and up-regulation of BDNF and muscarnic-1 receptor expression. Cell Mol Neurobiol 33: 875-884.
- Pandareesh MD, Anand T (2014) Neuroprotective and anti-apoptotic propensity of Bacopa monniera extract against sodium nitroprusside induced activation of iNOS, heat shock proteins and apoptotic markers in PC12 cells. Neurochem Res 39: 800-814.
- Pandareesh MD, Anand T (2014) Attenuation of smoke induced neuronal and physiological changes by bacoside rich extract in Wistar rats via down regulation of HO-1 and iNOS. Neurotoxicol 40: 33-42.
- Bharali DJ, Mousa SA (2010) Emerging nanomedicines for early cancer detection and improved treatment: current perspective and future promise. Pharmacol Ther 128: 324-335.
- Siddiqui IA, Adhami VM, Bharali DJ, Hafeez BB, Asim M, et al. (2009) Introducing nano-chemoprevention as a novel approach for cancer control: proof of principle with green tea polyphenol epigallocatechin-3-gallate. Cancer Res 69: 1712-1716.
- Srivastava AK, Bhatnagar P, Singh M, Mishra S, Kumar P, et al. (2013) Synthesis of PLGA nanoparticles of tea polyphenols and their strong in vivo protective effect against chemically induced DNA damage. Int J Nanomedicine 8: 1451-1462.
- Xie X, Tao Q, Zou Y, Zhang F, Guo M, et al. (2011) PLGA nanoparticles improve the oral bioavailability of curcumin in rats: characterizations and mechanisms. J Agric Food Chem 59: 9280-9289.
- Sanna V, Siddiqui IA, Sechi M, Mukhtar H (2013) Resveratrol-loaded nanoparticles based on poly(epsilon-caprolactone) and poly (d,l-lactic-co-glycolic acid)-poly(ethylene glycol) blend for prostate cancer treatment. Mol Pharm 10: 3871-3881.
- Montgomery DC (2005) Design and Analysis of Experiments Response Surface Method and Designs; John Wiley and Sons: New Jersey, USA.
- Box GEP, Draper NR (1987) Empirical Model Building and Response Surface. Wiley: New York.
- Yadav DN, Sharma GK, Bawa AS (2007) Optimization of soy-fortified instant sooji halwa mix using response surface Methodology. J Food Sci Tech 44: 297-300.
- Jalarama KR, Pandey MC, Harilal PT, Radhakrishna K (2013) Optimization and quality evaluation of freeze dried mutton Manchurian. Int J Food Res 20: 3101-3106.
- Jalarama KR, Jayathilakan K, Pandey MC (2015) Development of designer chicken shred with response surface methodology and evaluation of its quality characteristics. J Food Sci Tech 53: 471-480.
- Madamba PS (2002) The response surface methodology: an application to optimize dehydration operations of selected agricultural crops. Lebensittel- Wissenschaft and Technology (LWT) 35: 584-592.
- Madahar GS, Toledo RT, Florod JD, Jen JT (1989) Optimization of carrot dehydration process using response surface methodology. J Food Sci 54: 714-719.
- Myers RH, Montegomery DC (2002) A Text book of “Response surface methodology: Process and product optimization using design experiments, (2nd edn), John Wiley & Sons Inc; USA, pp: 321-342.
- Sin HN, Yusof S, Sheikh AHN, Rahman AR (2006) Optimization of hot water extraction for sapodilla juice using response surface methodology. J Food Eng 74: 352-358.
- Watoo P, Sakchal W, Kanchalee J, Ubrapron P, Hiroyuki T, et al. (2007) Determination of sopanin glycosides in Bacopa monniera by reverced phase high performance liquid chromatography. Thai Pharm Health Sci J 2: 26-32.
- Eberhardt MV, Lee CY, Liu RH (2000) Antioxidant activity of fresh apple. Nature 405: 903-904.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic acid-phosphotungstic acid reagents. Am J Enol Vit 16: 144-158.
- Cai Y, Luo Q, Sun M, Corke, H (2004) Antioxidant activity and phenolic compounds of 112 traditional Chinese medicinal plants associated with anticancer. Life Sciences 74: 2157-2184.
- Crissman HA, Steinkamp JA (1973) Rapid Simultaneous measurement of DNA, protein and cell volume in single cells from a large mammalian cell population. J Cell Biol 59: 766-771. [Crossref]
- Pillai CKS, Paul W, Sharma CP (2009) Chitin and chitosan polymers: chemistry, solubility and fibre formation. Prog Polymer Sci 34: 641-678.
- Bahreini E, Aghaiypour K, Abbasalipourkabir R, Mokarram AR, Goodarzi MT (2014) Preparation and nanoencapsulation of l-asparaginase II in chitosan-tripolyphosphate nanoparticles and in vitro release study. Nanoscale Res Lett 9: 340.
- Shah M, Behara YR, Jagadeesh B (2012) Phytochemical screening and in vitro antioxidant activity of aqueous and hydroalcoholic extract of Bacopa monniera. Linn International J Pharmaceut Sci Res 3: 3418-3424.