Background: Question arises if Amyotrophic lateral sclerosis (ALS, Lou-Gehrig-Syndrom, Charcot-disease) is considered to be an autoimmune disorder that affects the nerval filaments of α-motoneurons in the central nervous system (CNS) due to oxidative stress. SOD mutations seem to play a genetic disorder role in glycosylation and oxidative modifications of neuronal filaments. Vitamin D (VitD) and Vitamin D binding protein (VDBP) has potency to act as an immune modulator. The present study was undertaken to examine the effects of a 34-week vitD supplementation of ImmunoDÒ, a stabilized Vitamin D3 complex, in a patient with ALS on oxidative stress parameters, nitrosative stress parameter, VitD status and VDBP.
Results: This case study showed early beneficial responses, especially improvement of motor dysfunction after VitD treatment. During treatment lipid peroxidation products like malondialdehyde (MDA), 4-hydroxynonenal (HNE) and hydroxyalkenale (HAE) increased in the first 4 weeks (MDA: 1.00 µM to 4.36 µM; HNE: 1.18 µM to 4.78 µM; HAE: 2.18µM to 9.14 µM), but decreased after 8 and 34 weeks (MDA: 0.89 µM to 0.76 µM; HNE: 0.75 µM to 0.68 µM; HAE: 1.64 µM to 1.44 µM). Carbonyl proteins showed the same trend (0 weeks: 245 pmol/mg, 4 weeks: 392 pmol/mg, 8 weeks: 284 pmol/mg and 34 weeks: 211 pmol/mg but not total oxidative status and nitrated tyrosine proteins. VitD and VDBP increased after 34 weeks therapy compared before treatment (VitD before treatment: 150.4 ng/mL, after 34 weeks: 194.8 ng/mL; VDBP before treatment: 175.7 mg/mL, after 34 weeks: 225 mg/mL): Oxidative stress, VitD and VDBP seem to be involved in the pathogenesis of ALS.
carbonyl proteins (CP), lipid aldehydes (MDA, HNE), vitamin D, vitamin D binding protein (ImmunoD®), nitro-tyrosin-protein (NTP)
Abbreviation
CP: carbonyl proteins; NTP: nitro-tyrosin-protein
Amyotrophic lateral sclerosis (ALS) is a fatal neurodegenerative disease in which pathogenesis and death of motor neurons are triggered by non-cell-autonomous mechanisms [1]. Whereas, multiple sclerosis is one of the best-studied diseases and some of the information obtained from 25,000 patient-years of observation are publisched recently [2], the immunological mechanisms of amyotrophic lateral sclerosis (ALS) are only poorly understood. Amyotrophic lateral sclerosis (ALS) is characterized by degeneration of corticomotoneurons in both the cerebral cortex and the spinal cord [3-5]. The result is a syndrome of mixed upper and lower motoneuron signs which progresses relentlessly and leads to death at a median time of 29.1 months after presentation [6]. Based on 1,028 identified incident cases, the crude annual incidence rate of ALS in the general European population was 2.16 per 100,000 person-years; 95% CI 2.0-2.3), with similar incidence rates across all registries [7]. The incidence was higher among men (3.0 per 100,000 person-years; 95% CI = 2.8 to 3.3) than among women (2.4 per 100,000 person-years; 95% CI=2.2 to 2.6). Spinal onset ALS was more common among men compared to women, particularly in the 70-80 year age group. Disease occurrence decreases rapidly after 80 years of age [7]. Patients with bulbar onset ALS usually present with dysarthria and dysphagia for solid or liquids, and limbs symptoms can develop almost simultaneously with bulbar symptoms, and in the vast majority of cases will occur within 1-2 years. Paralysis is progressive and leads to death due to respiratory failure within 2-3 years for bulbar onset cases and 3-5 years for limb onset ALS cases [8].
There is a large amount of evidence implicating oxidative damage in disease pathogenesis of ALS. An increase in protein carbonyls was found in frontal cortex (Broadman area 6) and in motor cortex (Broadman area 4) [9]. A further study demonstates a higher formation of hydroxynonenal (HNE) modified protein by immunohistochemistry in ALS spinal cord motor neurons [10]. While most results of modified proteins and/or poly-unsaturated fatty acids in ALS are derived from post mortem immuno histochemical observations, only few reports showed a significant increase of break down products of poly unsaturated fatty acids like malondialdehyde (MDA) compared to healthy population in human serum [11]. Since there is no curative and only limited effective treatment options available for the patient, we performed this case study to elucidate whether vitamin D is therapeutically effective in the treatment of ALS accompanied by blood analysis of oxidative and nitrosative blood parameters like carbonyl proteins (CP), nitro-tyrosine proteins (NTP), MDA, HNE and hydroxyalkenale (HAE), lipid peroxides as marker of total oxidative status (TOS). We estimated also the VitD, VDBP and status during the therapy for the first time.
An Austrian male medical general practitioner developed ALS confirmed by anamnesis, ECG, EMG and magnetic resonance imaging (MRI) scan of the brain in 2015 at the age of 63.
First symptoms with dyarthria appeared in Febraury 2015.Progredient neurological symptoms with further bulbar dysarthria, dysphagia and parästhesia in right leg starting from toe 5 to 1. The tongue showed marginal athropic signs and reduced motility. The movement of the right hand was very limited and made eating with this hand impossible with partia athropic muscles. He had to stop working as a doctor and closed down his practice in October 2015.
He was treated with Rilutec 50 mg twice daily since march 2015 and oral Vitamine D 3000 IU daily.
From April 2016 he received 1mL Vitamin D3 complex (ImmunoD®, 400 IU Vitamin D3; HG Pharma) intravenously twice weekly. No adverse event or negative side effects were noticed by the patient. Venous blood was obtained before Vitamin D regiment, 4 weeks and 8 weeks after 34 weeks. After centrifugation (2000 rpm), the plasma was aliquoted and stored at -70°C before measurements.
Vitamin D3 complex (ImmunoD® ) was obtained from HG Pharma (Vienna, Austria).
Oxidative and nitrosative stress blood plasma anaylsis
Measurement of CP was performed by the CP-ELISA (Immundiagnostik AG, Bensheim, Germany). Plasma samples and standards were diluted to give a final protein concentration of 0.4 mg/ml. Measurement of CP was performed after derivatization with dinitrophenyl-hydrazine (DNPH) [12]. Plasma protein was measured with the bicinchoninic assay (BCA; Pierce, Rockford, USA).
The colorimetric assay for lipid peroxidation product (Oxford Biomedical Research, Oxford, USA) has been used as an indicator of lipid peroxidation [13-14].
This method is designed to assay either MDA alone or MDA in combination with 4-hydroxyalkenals. This assay is based on the reaction of a chromogenic reagent with MDA and 4-hydroxyalkenals at 45°C with maximal absorbance at 586 nm.
Oxidized LDL (oxLDL) was performed by the ox-LDL/MDA adduct ELISA kit from Immundiagnostik (Immundiagnostik AG, Bensheim, Germany) as described in a recent study [15]. Standards, controls and sample containing human oxLDL were added to wells of a microplate coated with high affinity antibodies. During the first incubation period, the antibodies captured the antigen of samples. After washing away unbound components from samples, a peroxidise-conjugated antibody was added to each microtiter well. Finally, after incubation and washing the microplate 3,3',5,5'tetramethylbenzidine solution (TMB) was used as a substrate for peroxidase. Incubation with TMB was terminated by adding an acid solution. The intensity of the yellow colour is directly proportional to the oxLDL concentration. A dose response curve of absorbance unit (optical density at 450nm) versus concentration was generated and oxLDL present in the patient samples were calculated.
For the in vitro determination of nitrotyrosine in human ETDA-plasma the ELISA Nitrotyrosine (NTP) from Immundiagnostik AG was used (Bensheim, Germany). Standards, controls and diluted samples which are assayed for nitrotyrosine are added into the wells of a micro plate coated with polyclonal goat anti- nitrotyrosine antibody. During the first incubation step, nitrated proteins are bound by the immo- bilized primary antibody. Then a peroxidase-conjugated polyclonal goat anti-human serum proteins antibody is added into each microtiter well and a “sandwich” of primary antibody - nitrated protein – peroxidase-conjugate is formed. Tetramethylbenzidine is used as peroxidase substrate. Finally, an acidic stop solution is added to terminate the reaction. The color changes from blue to yel- low. The intensity of the yellow color is directly proportional to the concentration of nitrotyrosine. A dose response curve of the absorbance unit (optical density, OD at 450 nm) vs. standard concentration is generated, using the values obtained from the standards.
Measurement of 25(OH)-Vitamin D and Vitamin Binding Protein (VDBP)
25(OH)-Vitamine D direct day is intended for the quantitative determination of the 25-OH-Vitamin D in serum and fresh plasma (Immundiagnostik AG, Bensheim, Germany). The assay utilizes of a competitive ELISA technique with a selected monoclonal anti- body recognizing 25(OH)-vitamin D. For a reliable determination of 25(OH)-vitamin D, it is necessary to release it from the 25(OH)-vitamin D-VDBP-complex. Standards, controls and patient samples which are assayed for 25(OH)-vitamin D are prediluted with the releasing reagent and transferred to the microplate coated with 25(OH)-vitamin D. After an incubation to release the 25(OH) vitamin D, an anti- 25(OH)-vitamin D antibody is added. During an incubation step, 25(OH)-vitamin D in the sample and a fixed amount of 25(OH)-vitamin D bound to the microtiter well compete for the binding of the antibody. Then a peroxidase-conjugated antibody is added into each microplate well. A complex of 25(OH)-vitamin D – anti-25(OH)- vitamin D antibody – peroxidase conjugate is formed. Tetramethylbenzidine (TMB) is used as a peroxidase substrate. Finally, an acidic stop solution is added to terminate the reaction, whereby the color changes from blue to yellow. The intensity of the yel- low color is inversely proportional to the concentration of 25(OH)-vitamin D. A dose response curve of the absorbance unit (optical density, OD at 450 nm) vs. concentra- tion is generated using the values obtained from the standard. 25(OH)-vitamin D in the samples is determined from this curve.
The Vitamin D-Binding Protein ELISA kit is a sandwich enzyme immunoassay for in vitro quantitative measurement of DBP in Plasma (Antibodies-online Inc., Atlanta, USA). This assay has high sensitivity and excellent specificity for detection of Vitamin D Binding Protein (DBP). The microtiter plate provided in this kit has been pre-coated with an antibody specific to DBP. Standards or samples are then added to the appropriate microtiter plate wells with a biotin-conjugated antibody preparation specific for DBP. Next, Avidin conjugated to Horseradish Peroxidase (HRP) is added to each microplate well and incubated. After TMB substrate solution is added, only those wells that contain DBP, biotin-conjugated antibody and enzyme-conjugated Avidin will exhibit a change in color. The enzyme-substrate reaction is terminated by the addition of sulphuric acid solution and the color change is measured spectrophotometrically at a wavelength of 450 nm ± 10 nm. The concentration of DBP in the samples is then determined by comparing the O.D. of the samples to the standard curve.
Quality of life questionaire (QoL) by Short Form (36) Health Survey (SF-36)
The Short Form (36) Health Survey is a 36-item, patient-reported survey of patient health. The SF-36 is a measure of health status under treatment [16] . The german version was evaluated by Bullinger et al. In 1998 [17-18]. The RAND Short Form 36 (SF-36) was used to measure patient Quality of Life (QoL) during application of ImmunoD. It measures QoL across eight emotional and physical domains: physical functioning; role limitations due to physical health; role limitations due to emotional problems; energy/fatigue; emotional well-being; social functioning; pain; general health. The questionaire was filled out by the patient prior to therapy, after five weeks of treatment and after therapy of 38 weeks. Evaluation of results was calculated using the automated web based SF-36 Survey App (ÓMedia GmbH, Potdam, Germany, Version: 1.3 – 2013-03-19). An increase in the scores marks an improvement in QoL. The scoring of the SF-36 indicates that 0% in a domain represents the poorest possible QoL and 100% indicates full QoL (the best possible result).
After ten weeks of treatment the following beneficial responses were observed; a.) The paraesthesia of the right hands was only limited to finger four and five. b.) Only slight motoric weakness of the hand was left and was only mentioned while doing heavy work with the hand, like turning a key. c.) The paresthesia of the right leg disappeared completely. d.) He noticed only slight dysphagia and very mild dysarthria. e.) The treatment with Rilutek could be reduced to 50 mg once daily. f.) The patient was able to perform a bicycle tour through central italy. That was the first time since years. g.) He is now able to frequently doing hiking tours through the austrian mountains.
Quality of life questionaire (QoL) by Short Form (36) Health Survey (SF-36)
Differences in QoL were measured using the standardized SF-36 questionaire. We used the SF-36 Questionaire to evaluate the change in QoL in an ALS patient under ImmunoD treatment to figure out the personal QoL enhancement and effectiveness of the product. The measurement is an expression of the personal QoL before, during and after treatment. Data were collected using the filled out standardized german SF-36 quesionaire. The questionaire was filled out by the patient at the described time points without any influence by the investigator. The calculated summarized increase in score was from 27,88% to 55%. Except of the measurement of emotional role (RE), an increase in each domain could be noticed. The biggest increase was recorded in RP, VT, SF and MH. A slight increase was also noted in PF, BP and GH. RP increased from 0% to 25%, VT from 0% to 60%, SF from 12,5% to 50% and MH from 8% to 80%. PF increased from 90% to 95%, BP from 87,5% to 100% and GH from 25% to 30% after treatment. Measurements for RE was before and after treatment 0%. The orange line in (Figure 1) demonstrates normal values. It can be demonstrated that the patient reached normal or almost normal values for PF, VT and MH after 34 weeks of treatment.
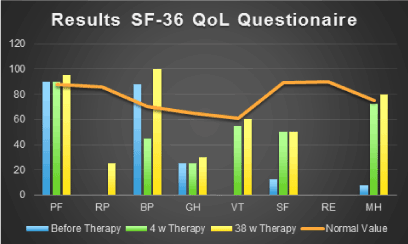
Figure 1: Demonstrates that the patient reached normal or almost normal values for PF, VT and MH after 34 weeks of treatment. (PF: Physical Functioning, RP: Role Physical, BP: Bodily Pain, GH: General Health, VT: Vitality, SF: Social Functioning, RE: Role Emotional, MH: Mental Health).
Oxidative stress blood analysis (Table 1)
Table 1. Oxidative stress blood analysis
| |
Therapy time (weeks) |
|
|
0 |
4 |
8 |
34 |
Reference |
CP |
245 |
392 |
284 |
211 |
0 - 200 µmol/mg |
NTP |
341.5 |
180.4 |
402.4 |
397.5 |
0 - 549 ng/mL |
VitD |
150.4 |
110.4 |
65.8 |
194.8 |
30 - 80 ng/mL |
TOS |
177.2 |
34.8 |
58.4 |
97.8 |
0 - 200 µM |
MDA |
1.00 |
4.36 |
0.89 |
0.76 |
0 - 0.9 µM |
HNE |
1.18 |
4.78 |
0.75 |
0.68 |
0 - 0.67 µM |
HAE |
2.18 |
9.14 |
1.64 |
1.44 |
0 - 1.57 µM |
Carbonyl proteins in serum were increased before VitD regiment (245 IU/mL), after 4 weeks therapy CP reached maximum with 392 IU/mL. After 8 and 34 weeks of therapy the amount of CP decreased to 392 IU/mL and 211 IU/mL. Free carbonyls, like MDA, HNE and HAE were also analyzed, which are able to interact with proteins forming CP. In all 3 parameters MDA, HNE and HAE we can see the same trend like CP. Before treatment MDA was within the normal range (1.00 µM) but increased after 4 weeks up to 4.36 µM. After additionally 8 weeks the amount of MDA decreased to 0.89 µM where it nearly remained after 34 weeks (0.76 µM). Both estimations were within the normal range. Compared to MDA, HNE and HAE were higher than the normal range at the beginning of therapy (HNE: 1.18 µM; HAE: 2.18 µM). Both carbonyls rose up after 4 weeks therapy (HNE: 4.78 µM; HAE: 9.14 µM). After 8 weeks HNE (0.75 µM) and HAE (1.64 µM) decreased and further reduction was obtained after 34 weeks (HNE: 0.68 µM; HAE: 1.44 µM). While HAE was within the normal range after 34 weeks, HNE was slightly increased compared to a healthy control group.
Free carbonyl groups are generated from lipid peroxides of oxidized poly-unsaturated fatty acids. Lipid peroxides are measured by the Total Oxidative Status test (TOS). All estimated concentrations of TOS before and during the therapy were within the normal range (before therapy: 177.2 µM; after 4 weeks: 34.8 µM; 58.4 µM; after 8 weeks: 58.4 µM; after 34 weeks: 97.8 µM), but the content of lipid peroxides decreased after 4, 8 and 34 weeks compared to the begin of the therapy.
At least the content of nitro-tyrosine bound protein (NTP) was measured. All estimated NTP concentrations were within the normal range and showed no significant difference (0 weeks: 341.5 weeks: 380.4 ng/mL; 8 weeks: 402.4 ng/mL; 34 weeks: 397.5 ng/mL).
Quantification of VitD and VDBP in human plasma (Table 2)
Table 2. Quantification of VitD and VDBP in human plasma
| |
Therapy time (weeks) |
|
|
0 |
8 |
34 |
Reference |
VitD |
150.4 |
65.8 |
194.8 |
30 - 80 ng/mL |
VDBP |
175.7 |
58.7 |
225 |
mg/mL |
VitD/VDBP |
0.086 |
0.112 |
0.087 |
‰ |
During treatment we measured also the VitD status and the content of VDBP before, 8 week and 34 week VitD treatment. Before treatment was nearly double higher than the normal range (150.4 ng/mL). After 8 weeks treatment VitD content increased slightly 110.4 ng/mL, after 34 weeks up to 194.8 ng/mL. The same trend was measured with VDBP: Before treatment the concentration was 175.7 mg/mL, after 8 weeks 158.7 mg/mL and at least after 34 weeks 225 mg/mL.
We calculated also the VitD/VDBP ratio during the regiment. Before and after 34 weeks of vitamin D therapy the ratio was 0.086 and 0.087%. Only after 4 weeks therapy the VitD/VDBP ratio increased up to 0.98%.
ALS is a fatal neurodegenerative disease characterized by progressive motor dysfunction and loss of large motor neurons in the spinal cord and brain stem. While much research has focused on mechanisms of motor neuron cell death in the spinal cord, degenerative processes in skeletal muscle and neuromuscular junctions (NMJs) are also observed early in disease development [19].
ALS and multiple sclerosis (MS) are neurodegenerative diseases that impact the central nervous system. Both diseases attack the body’s nerves and muscles and in many ways, these two diseases are very similar. People with MS often experience greater mental impairment than people with ALS. People with ALS typically develope greater physical difficulties. ALS is not an autoimmune disease and its cause is largely unknown. One postulated origin is a disorganized immune response. According to the National Institute of Neurological Disorders and Stroke, eventually all individuals with ALS will become unable to walk, stand, or move about without help. They also may develop great difficulty swallowing and chewing. Ultimately, ALS is fatal. ALS commonly starts in the hands, feet, or arms and legs. It then spreads to other parts of the body. It does not affect your thought processes or senses. However, later stages can include dementia.
As with MS, there is not a cure for ALS. Treatments are to slow symptoms and prevent some complications, Riluzole (Rilutek) is the only FDA-approved drug to treat ALS. For some, it seems to slow the disease’s progression. However, there are other drugs that can help manage other symptoms like constipation, fatigue, and pain [20]. Currently, there is no curable therapy option.
In this pilot investigation, Vitamin D3 complex, as the modulator of the immune system, exhibited potent therapeutic activity for ALS. In particular, physical disability in ALS was drastically improved following the administration of Vitamin D3 complex, indicating that the treatment of Vitamin D3 complex promoted the incentive of physical rehabilitation in ALS with emotional disorders as mentioned above. Moreover, although, at this time, it is not clear how Vitamin D3 complex precisely modulates the immune system and what the therapeutic mechanism of Vitamin D3 complex is, it seems likely that Vitamin D3 complex may influence the imbalance of the immune system and emotional comorbidities.
Recently, we reported about the important role of oxidative stress in neurodegenerative diseases [21-23] the potential anti-inflammatory modulation of their progress [24]. We aimed to investigate oxidative stress biomarkers in this ALS patient during Vit D therapy because ALS is associated with SOD1 mutation and therefore higher oxidative and nitrosative modifications of neuronal cells. Lipid peroxides are generated by free radicals, like superoxide anion radicals reacting with poly unsaturated fatty acids (PUFAs). Lipid peroxides are intermediate breakdown products of lipid peroxidation which itself generates toxic lipid aldehydes like MDA, HNE and HAE. In our case report the amount of lipid peroxides (TOS) is in the reference range before, during and after the Vitamin D3 complex therapy. This indicates eventually no active lipid peroxidation of PUFAs. Interestingly, MDA and further aldehydes (HNE, HAE) are increased before the therapy. This is in consent with the findings of Fang et al. [11]. During therapy MDA but also HNE and HAE increased after 4 weeks extensively because of immune stimulation, whereas after 8 and 34 therapy weeks they decreased nearly within the reference range. The same progression was measured also on CP. Higher CP was reported by Bowling et al. in ALS patients [25]. CP are generated (i) directly by radicals, (ii) metals,(iii) lipid peroxidation or (iv) O-glycoxidation.
2021 Copyright OAT. All rights reserv
We have also estimated the amount of VitD during the therapy. The ALS patient took before, during and after the Vitamin D3 complex therapy additionally 3000 I.U. of VitD orally. Before the therapy the VitD level was increased and over the reference range. After 4 and 8 weeks the VitD level decreased within the reference range, whereas after 34 weeks VitD arised. The same situation we could observe with VDBP. Calculating the ratio of Vit.D/VDBP we detected no relevant change before and after therapy, but it has to be noted that only small amounts of VitD are transported by VDBP.
In summary this case study demonstrated that treatment with Vitamin D3 complex markedly improved the motor disability in a patient with ALS, suggesting the possibility that VitD, an oxidative stress and immune modulator, is useful for the treatment of this disease.
Furthermore, a large-scale clinical study and experimental studies using the animal model would be required to clarify the definitive efficacy of VitD in ALS and the probable mechanism(s) for the pathophysiological actions of VitD. Moreover, the examination of brain MRI, physical activity and neuropsychological evaluation will help elucidate further information on therapeutic efficacy of Vitamin D3 complex as Vit D therapy option in ALS.
- Rojas F, Gonzalez D, Cortes N, Ampuero E, Hernández DE, et al. (2015) Reactive oxygen species trigger motoneuron death in non-cell-autonomous models of ALS through activation of c-Abl signaling. Front Cell Neurosci 9: 203.
- Ebers GC (2000) The natural history of multiple sclerosis. Neurol Sci 21: S815-7.
- Brownell B, Oppenheimer DR, Hughes JT (1970) The central nervous system in motor neurone disease. J Neurol Neurosurg Psychiatry 33: 338-357.
- Lawyer T, netsky MG (1953) Amyotrophic lateral sclerosis. AMA Arch Neurol Psychiatry 69: 171-192.
- Smith MC (1960) nerve fibre degeneration in the brain in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 23: 269-282.
- Munsat TL, Andres PL, Finison L, Conlon T, Thibodeau L, et al. (1998) The natural history of motoneuron loss in amyotrophic lateral sclerosis. Neurology 38: 409-413.
- Logroscino G, Traynor BJ, Hardiman O, Chio A , Mitchell D, et al. (2010) Incidence of amyotrophic lateral sclerosis in Europe. J Neurol Neurosurg Psychiatry 81: 385-390.
- Wijesekera LC, Leigh PN (2009) Amyotrophic lateral sclerosis. Orphanet J Rare Dis 4: 3.
- Ferrante RJ, Browne SE, Shinobu LA, Bowling AC, Baik MJ, et al. (1997) Evidence of increased oxidative damage in both sporadic and familial amyotrophic lateral sclerosis. J Neurochem 69: 2064-2074.
- Pedersen WA, Fu W, Keller JN, Markesbery WR, Appel S, et al. (1998) Protein modification by the lipid peroxidation product 4-hydroxynonenal in the spinal cords of amyotrophic lateral sclerosis patients. Ann Neurol 44: 819-824.
- Fang L, Huber-Abel F, Teuchert M, Hendrich C, Dorst J, et al. (2009) Linking neuron and skin: matrix metalloproteinases in amyotrophic lateral sclerosis (ALS). J Neurol Sci 285: 62-66.
- Lamprecht M, Greilberger J, Oettl K (2004) Analytical aspects of oxidatively modified substances in sports and exercises. Nutrition 20: 728-730.
- Esterbauer H, Schaur RJ, Zollner H (1991) Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med 11: 81-128.
- Lamprecht M, Bogner S, Schippinger G, Steinbauer K, Fankhauser F, et al. (2012) Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. J Int Soc Sports Nutr 9: 45.
- Matzi V, Greilberger JF, Lindenmann J, Neuboeck N, Nuhsbaumer S, et al. (2015) Application of Hyperbaric Oxygen Reduce Oxidative Damage of Plasmatic Carbonyl Proteins and 8-OHdG by Activating Glutathion Peroxidase. Clin Lab 61: 587-593.
- Ellert U, Bellach BM (1999) The SF-36 in the Federal Health Survey--description of a current normal sample. Gesundheitswesen 61: S184-90.
- Bullinger M, Azouvi P, Brooks N, Basso A, Christensen AL, et al. (2002) Quality of life in patients with traumatic brain injury-basic issues, assessment and recommendations. Restor Neurol Neurosci 20: 111-124.
- Bullinger M, Blome C, Sommer R, Lohrberg D, Augustin M, et al. (2015) Health-related quality of life: a pivotal endpoint in benefit assessment of medical procedures. Bundesgesundheitsblatt Gesundheitsforschung Gesundheitsschutz 58: 283-290.
- Van Dyke JM, Smit-Oistad IM, Macrander C, Krakora D, Meyer MG, et al. (2016) Macrophage-mediated inflammation and glial response in the skeletal muscle of a rat model of familial amyotrophic lateral sclerosis (ALS). Exp Neurol 277: 275-282.
- Geevasinga N, Menon P, Ng K, Van Den Bos M, Byth K, et al. (2016) Riluzole exerts transient modulating effects on cortical and axonal hyperexcitability in ALS. Amyotroph Lateral Scler Frontotemporal Degener 17: 580-588
- Rommer PS, Greilberger J, Salhofer-Polanyi S, Auff E, Leutmezer F, et al. (2014). Elevated levels of carbonyl proteins in cerebrospinal fluid of patients with neurodegenerative diseases. Tohoku J Exp Med 234: 313-317.
- Greilberger J, Fuchs D, Leblhuber F, Greilberger M, Wintersteiger R, et al. (2010) Carbonyl proteins as a clinical marker in Alzheimer’s disease and its relation to tryptophan degradation and immune activation. Clin Lab 56: 441-448.
- Greilberger J, Koidl C, Greilberger M, Lamprecht M, Schroecksnadel K, et al. (2008) Malondialdehyde, carbonyl proteins and albumin-disulphide as useful oxidative markers in mild cognitive impairment and Alzheimer’s disease. Free Radic Res 42: 633-638.
- Rommer PS, Fuchs D, Leblhuber F, Schroth R, Greilberger M, et al. (2016) Lowered Levels of Carbonyl Proteins after Vitamin B Supplementation in Patients with Mild Cognitive Impairment and Alzheimer’s Disease. Neurodegener Dis 16: 284-289.
- Bowling A, Schulz JB, Brown RH Jr, Beal MF (1993) Superoxide dismutase activity, oxidative damage, and mitochondrial energy metabolism in familial and sporadic amyotrophic lateral sclerosis. J Neurochem 61: 2322-2325.

