Patients with inoperable pancreatic adenocarcinoma have limited options, with conventional chemoradiation offering modest survival benefit. Image-guided stereotactic ablative radiotherapy (SABR) has shown promise in delivering large conformal radiation doses to the pancreas safely while sparing the adjacent luminal gastrointestinal tract. Retrospective studies have suggested that the use of SABR in treating inoperable pancreatic cancer due to locally advanced disease (LAPC) may increase local control and thus improve overall survival. Here we present a novel case report of an elderly patient with LAPC who was successfully treated with image-guided SABR alone. Following SABR, the patient showed a sustained complete remission for over 78 months with acute and late toxicity induced by radiation therapy being limited to Grade 2. This case report may help to expand the role of image-guided SABR with dose escalation of boost area as a salvage treatment in LAPC. Further cases and prospective clinical studies are needed to verify the efficacy.
Mini Abstract
We present a patient with LAPC following SABR, with complete remission 78 months, which showed SABR with dose escalation of boost area may be a salvage treatment in LAPC.
Introduction
Pancreatic cancer is the sixth and third leading cancer death cause in China and the United States, respectively [1-3]. Radical resection has been considered the only curative option of achieving long-term survival with 5-year overall survival (OS) rates of 20% to 25% [4]. However, the condition in the majority of patients with pancreatic cancer is unresectable at the diagnosis, mainly due to distant metastases or locally advanced disease [5]. For patients with locally advanced pancreatic cancer (LAPC), the use of conventional radiation therapy (RT) combined with concurrent chemotherapy has shown mixed survival results in randomized clinical trials with a median OS from diagnosis of 8.6 to 15.2 months [6-8].
Stereotactic ablative radiotherapy (SABR) with image guidance has recently been developed as a system for delivering conformal high doses of radiation to pancreatic tumors, with simultaneous sparing of the adjacent luminal gastrointestinal tract [4,9,10]. Several retrospective studies [3,11,12] have suggested that the use of image-guided SABR after initiating chemotherapy in treating LAPC may increase local control and thus improve OS. As yet, no study has directly compared image-guided SABR with conventional chemoradiation or SABR with chemotherapy alone. Thus it is difficult to rule out the possible confounding effect of salvage chemotherapy on survival. More recently, SABR has been recommended for patients with LAPC who are elderly or have a decline in the Eastern Cooperative Oncology Group performance status (ECOG) in the American Society of Clinical Oncology clinical practice guideline [13,14]. In this case report, we present an elderly patient with LAPC who showed a sustained complete remission for over 78 months following image-guided SABR solely.
Case presentation
A 66-year-old Chinese male presented to our institution in April 2013 with newly diagnosed, undifferentiated carcinoma of the pancreas (Figure 1). On April 18, 2013, the magnetic resonance imaging (MRI) of the abdomen revealed a 7.1*4.5 cm neoplasm at the head and neck of pancreas and celiac trunk aneurysm, which was less than 1 cm luminal abutment (Figure 2a). Carbohydrate antigen 19-9 (CA-199) was 35.15 U/ml. He did not receive any treatment, and suffered persistent stomach bloating and intermittent abdominal distension, with the ECOG performance status of 2 (i.e., limitations in carrying out certain activities) [15]. The patient had no history of other comorbidities except for hypertension. A gastroscopy revealed duodenitis and multiple gastric ulcers, which ranged between 3 mm and 1.0 cm in diameter and were confirmed as chronic inflammation by biopsy (Figure 3a). Based on radiological findings, a multidisciplinary tumor conference consensus was that this was an inoperable LAPC because the cancer involved the celiac trunk artery and main portal vein with the former theoretically requiring arterial resection and reconstruction [5].
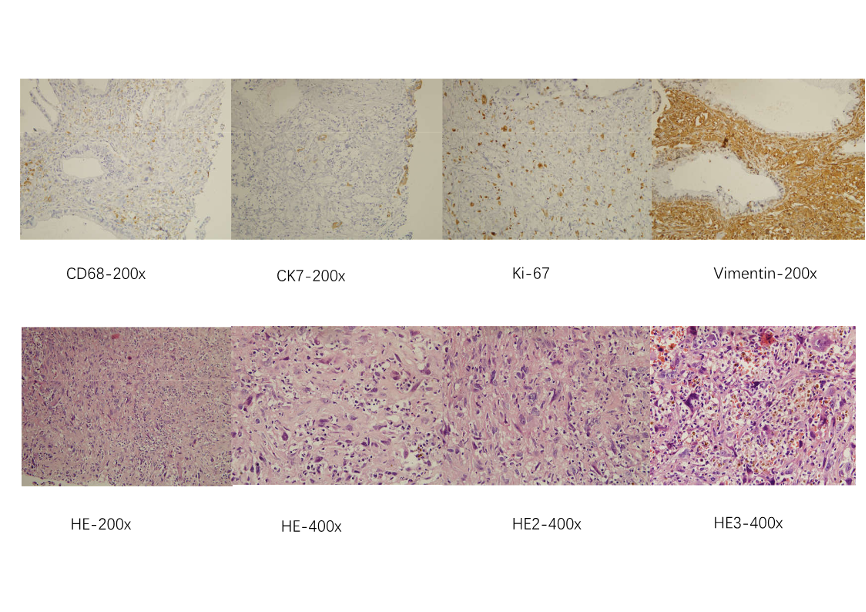
Figure 1. Carcinoma of the pancreas
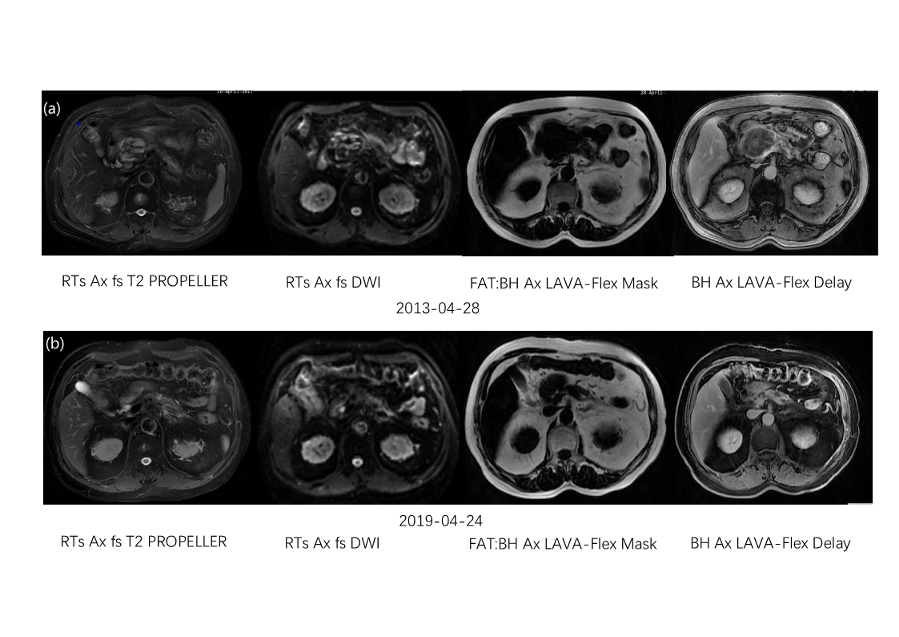
Figure 2. Luminal abutment

Figure 3. Chronic inflammation by biopsy
On April 28, 2013, the patient underwent SABR with helical tomotherapy technology. The ablative radiation delivery was computed tomography (CT)–based, image-guided hypofractionation, which delivered 20 fractions using intensity-modulated radiation and motion management. The abdominal compression measures for respiratory motion management, body mask and intravenous enhancing contrast were involved in CT simulation (Table 1). The macro-scopic (gross) tumor volume (GTV) was delineated on the fusion of simulation CT and MRI with the flu-orodeoxyglucose-positron emission tomography (FDG-PET/CT) images being the reference (Figure 4). The clinical target volume (CTV) was defined as 5mm margin expansion of the GTV and the final planning target volume (PTV) was a 3mm margin expansion of the CTV. In addition to the above target volumes, we delineated a small boost area named iGTV with a 2mm margin expansion of the GTV (Figure 5). Regional (peripancreatic) lymphnodes were included in the PTV. The prescription dose for PTV, CTV, GTV (biologically effective dose [BED] assuming α/β=10, BED10 = 94.5Gy) and iGTV (assuming α/β=10, BED10 = 112Gy) were 50, 60, 70 and 80Gy in 20 fractions delivered over 4 consecutive weeks, respectively. During radiotherapy, conventional treatments including hepatoprotective therapy, antiemetic drugs, and acid suppressive therapy were given. Concomitant nutritional support was not administered. After SABR, the patient opted to routine examination but refused adjuvant chemotherapy owing to toxicity concerns. Within three months of SABR, the acute toxicity induced by radiation therapy was assessed as Grade 2 according to the criteria of the Radiation Therapy Oncology Group (RTOG). CA-199 was 22.92 U/ml.
Simulation |
Treatment Planning (Dose Constraints) |
CT scan |
Proximal* duodenum, stomach, small bowel |
Siemens Emotion 16 |
D1 ≤ 55 Gy |
Abdominal enhanced CT scan |
D3 ≤ 50 Gy |
A calm breathing state |
D5 ≤ 45 Gy |
Thin-slice CT scan |
Liver: |
No food 2 h prior |
V20 ≤ 40% |
Supine position |
Combined kidneys |
Contrast |
V30 ≤ 30% |
Oral contrast: omnipaque (250 cc) |
Spinal cord |
Intravenous contrast: omnipaque (100 cc) |
Dmax ≤ 45 Gy |
Immobilization device |
|
a carbon fiber body-fixed frame |
|
thermoplastic films |
|
*Proximal defined as within 1 cm above and below the planning treatment volume
Abbreviations: CT: computed tomography; Gy: gray; Dma: maximum dose;
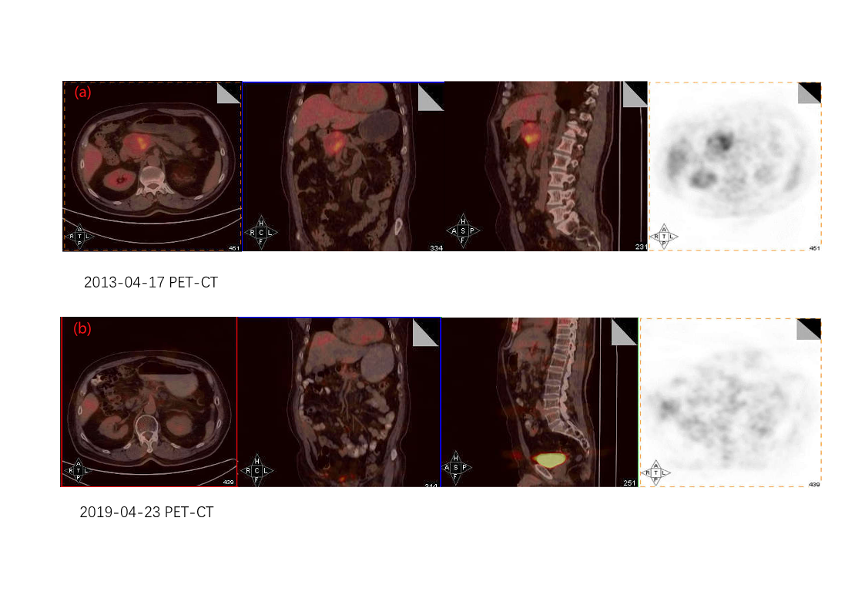
Figure 4. The macro-scopic (gross) tumor volume (GTV) was delineated on the fusion of simulation CT and MRI with the flu-orodeoxyglucose-positron emission tomography (FDG-PET/CT) images being the reference
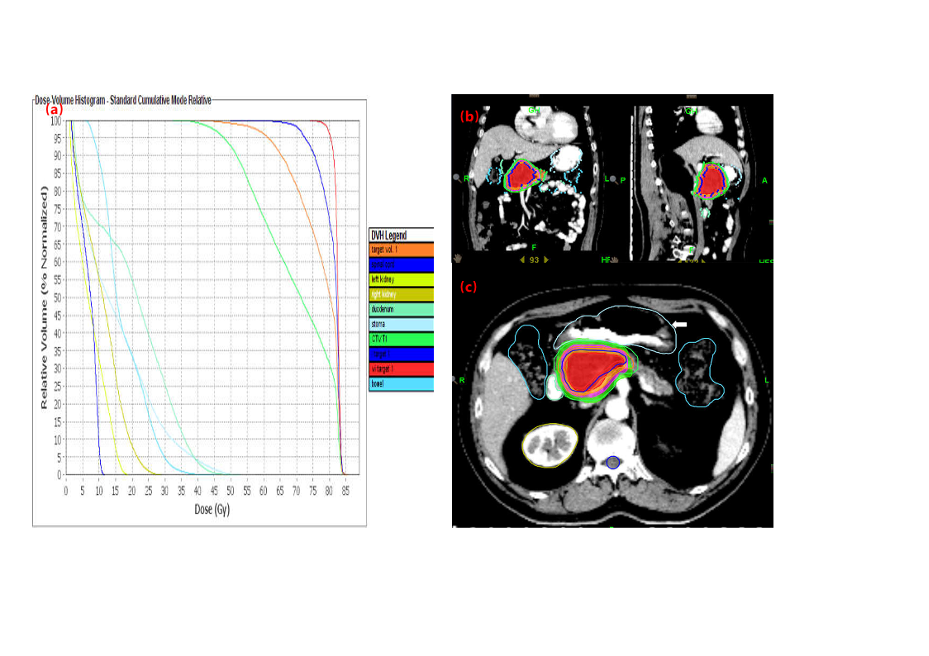
Figure 5. Expansion of the GTV
On April 22, 2019, the patient came back to the hospital because of epigastric pain. Other symptoms including abdominal pain, nausea, vomiting, loss of appetite, weight loss, and malaise, did not occur. Both MRI and PET/CT revealed the complete remission of primary pancreatic cancer (Figure 2b and Figure 4b). The gastroscopy revealed the size of the ulcers was similar to that observed prior to SABR with the diameters ranging from 0.3 to 1.0cm (Figure. 3b), which were confirmed as chronic inflammation by biopsy. The late toxicity was assessed as Grade 2. CA-199 was 14.40 U/ml.
On October 31, 2019, regional lymph node recurrence was confirmed by biopsy. The pathological type was undifferentiated carcinoma, which was the same as the original pancreatic cancer. Then the patient underwent 6 cycles of chemotherapy with Gemcitabine (1000 mg/m2 intravenously administered on days 1 and 8 of a 14-day cycle) and Tegafur Gimeracil Oteracil Potassium Capsule (40 mg twice daily, orally administrated every 7 days) in a local institute. On May 10, 2020, the patient underwent the last routine examination and was lost to follow up since then.
Up to date, the progression free survival (PFS) from the start of SABR was 78 months and OS was at least 84 months.
Discussion
Our case report represents the first reported inoperable pancreatic cancer case, who was treated with image-guided SABR with dose escalation of boost area and without concomitant chemotherapy, maintained 78 months of PFS and at least 84 months of OS from the start of SABR. The complete remission of primary pancreatic cancer after SABR is an extremely rare occurrence with no previous reports. This is a novel case of complete remission of inoperable pancreatic carcinoma following SABR as the sole treatment. While the pathophysiology to complete remission is not well understood and most likely to be multifactorial, the higher BED for booster area in our study than that in the previous studies may have contributed to a better local-regional control and a longer survival duration.
Five phase III randomized trials have shown that conventionally fractionated chemoradiation up to 60 Gy could produce a modest local control and add minimal to no survival benefit for patients with LAPC who have received chemotherapy [6-8,17,18]. While the high metastatic rate seen in this disease may contribute to, at least in part, the failure of a local control benefit translating into a survival benefit, it is also possible that the improvement in local control has not been significant enough to make a difference in survival [12,19-22], which underscores the need for further dose escalation.
With recent advancements and innovative solutions for planning and delivery of radiation therapy such as intensity modulated radiation therapy and image guided radiation therapy, it has become technically feasible to treat patients with LAPC with dose escalation [23-26]. Importantly, image-guided SABR with the greater number of fractions has not only enabled highly precise delivery of high doses of radiation to small tumor volumes [19], but also maintained an acceptable risk of toxicity for near radiosensitive gastrointestinal organs, primarily the duodenum, the jejunum and the stomach [19]. Furthermore, the central areas of a tumor are typically more hypoxic than the periphery and, therefore, more radioresistant and associated with a poor clinical prognosis secondary to treatment failure, relapse or metastasis [19]. As such, placing supra-ablative hotspots or boost area within the target volume of a more radioresistant portion of the tumor to achieve sharp dose gradients would be beneficial in reducing the recurrence of local malignancy and improving local-regional control [27-31].
Previous reports of dose escalation to an ablative threshold in retrospective single-institution cohorts suggested that controlling the primary tumor can improve survival [12,23,32-36]. Using 67.5 Gy in 15 or 75 Gy in 25 hypofractionated treatment regimens (BED of 98 Gy) in 119 patients with LAPC following the initial chemotherapy, median PFS and OS from the time of radiotherapy were 6.3 months (95% CI, 5.26-8.78) and 18.2 months (95% CI, 16.3-26.8), respectively [12,37-39]. In terms of median PFS and OS from diagnosis, they were 13.2 months (95% CI, 11.4-15.7) and 26.8 months (95% CI 21.7-35.7), respectively, with the latter comparing favorably with the 15.2 to [.5 months described in the 2 arms of the randomized LAP07 trial [6], as well as the results from a multi-institutional prospective phase 2 trial of 5-fraction SABR that gave a total dose of 33 Gy [35].
Although the patient had prevalent multiple gastric ulcers and duodenitis before SABR, the radiotherapy induced acute and late toxicity was limited to Grade 2 and no grade 3 to 5 gastrointestinal toxicity was observed after SABR. Multiple images fusion could help not only confirm the border of target, but also reduce the damage of adjacent organ at risk and prevent severe adverse effect.
For image guidance, MR guided radiotherapy, in which MR provides better contrast compared to CT, especially in gastrointestinal (GI) malignancies, may be a superior alternative to conventional on-board imaging [19]. However, when such technology is not accessible for patients and institutions, our approach of using optimized cone beam image-guidance delivered in 15 to 25 fractions with adaptive planning on as-needed basis could be more workflow-friendly [19].
The loss to follow-up after the regional recurrence was one of the major limitations in gaining a deep understanding of the complete history of treatment modalities. Chemotherapy was not applied after SABR to maximally reduce and stabilize the tumor. Because the patient continued to have subsequent treatment in a local institute but not in our hospital, we were unable to account for the effects of chemotherapy and obtain the actual OS time.
Conclusions
This teaching case illustrated that image-guided SABR with appropriate dose regimen may result in promising outcome in elderly patients with inoperable pancreatic cancer. Clinicians may need to pay more attention to the dose escalation of boost area in the GTV.
Author Responsible for Statistical Analysis Name & Email Address
N/A.
Conflict of Interest
None.
Funding Sources
This work is funded by Clinical Project of Air Force Medical Center,PLA (No:2021LC009) and Youth PhD Advancement Project of Air Force Medical Center,PLA(No:21ZT01).
Data Availability Statement for this Work
Research data are stored in an institutional repository and will be shared upon request to the corresponding author.
References
- Siegel RL, Miller KD, Fuchs HE, Jemal A (2022) Cancer statistics, 2022. CA Cancer J Clin 72: 7-33. [Crossref]
- Xia C, Dong X, Li H, Cao M, Sun D, et al. (2022) Cancer statistics in China and United States, 2022: profiles, trends, and determinants. Chin Med J (Engl) 135: 584-590. [Crossref]
- Rudra S, Jiang N, Rosenberg SA, Olsen JR, Roach MC, et al. Using adaptive magnetic resonance image-guided radiation therapy for treatment of inoperable pancreatic cancer. Cancer Med 8: 2123-2132. [Crossref]
- Petrelli F, Comito T, Ghidini A, Torri V, Scorsetti M, et al. (2017) Stereotactic Body Radiation Therapy for Locally Advanced Pancreatic Cancer: A Systematic Review and Pooled Analysis of 19 Trials. Int J Radiat Oncol Biol Phys 97: 313-322.
- Strobel O, Neoptolemos J, Jäger D, Büchler MW (2019) Optimizing the outcomes of pancreatic cancer surgery. Nat Rev Clin Oncol 16: 11-26. [Crossref]
- Hammel P, Huguet F, van Laethem JL (2016) Effect of Chemoradiotherapy vs Chemotherapy on Survival in Patients With Locally Advanced Pancreatic Cancer Controlled After 4 Months of Gemcitabine With or Without Erlotinib: The LAP07 Randomized Clinical Trial. JAMA 315: 1844-1853.
- Loehrer PJ Sr, Feng Y, Cardenes H, Wagner L, Brell JM, et al. (2011) Gemcitabine alone versus gemcitabine plus radiotherapy in patients with locally advanced pancreatic cancer: an Eastern Cooperative Oncology Group trial. J Clin Oncol 29: 4105-4112. [Crossref]
- Chauffert B, Mornex F, Bonnetain F, Rougier P, Mariette C, et al. (2008) Phase III trial comparing intensive induction chemoradiotherapy (60 Gy, infusional 5-FU and intermittent cisplatin) followed by maintenance gemcitabine with gemcitabine alone for locally advanced unresectable pancreatic cancer. Definitive results of the 2000-01 FFCD/SFRO study. Ann Oncol 19: 1592-1599. [Crossref]
- Horowitz DP, Goodman K, Kachnic LA (2021) Ablative Radiotherapy for Patients With Inoperable Pancreas Cancer-Ready for Prime Time? JAMA Oncol 7: 687-688. [Crossref]
- Qing S, Gu L, Zhang H (2021) Phase I study of dose-escalated stereotactic body radiation therapy for locally advanced pancreatic head cancers: Initial clinical results. Cancer Med 10: 6736-6743. [Crossref]
- Hassanzadeh C, Rudra S, Bommireddy A, Hawkins WG, Wang-Gillam A, et al. (2020) Ablative Five-Fraction Stereotactic Body Radiation Therapy for Inoperable Pancreatic Cancer Using Online MR-Guided Adaptation. Adv Radiat Oncol 6: 100506. [Crossref]
- Reyngold M, O'Reilly EM, Varghese AM, Fiasconaro M, Zinovoy M, et al. (2021) Association of Ablative Radiation Therapy With Survival Among Patients With Inoperable Pancreatic Cancer. JAMA Oncol 7: 735-738. [Crossref]
- Balaban EP, Mangu PB, Khorana AA, Shah MA, Mukherjee S, et al. (2016) Locally Advanced, Unresectable Pancreatic Cancer: American Society of Clinical Oncology Clinical Practice Guideline. J Clin Oncol 34: 2654-2668. [Crossref]
- Rosati LM, Herman JM (2017) Role of Stereotactic Body Radiotherapy in the Treatment of Elderly and Poor Performance Status Patients With Pancreatic Cancer. J Oncol Pract 13: 157-166. [Crossref]
- Oken MM, Creech RH, Tormey DC, Horton J, Davis TE, et al. (1982) Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5: 649-655. [Crossref]
- Cox JD, Stetz J, Pajak TF (1995) Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC). Int J Radiat Oncol Biol Phys 31: 1341-1346. [Crossref]
- Gastrointestinal Tumor Study Group (1988) Treatment of locally unresectable carcinoma of the pancreas: comparison of combined-modality therapy (chemotherapy plus radiotherapy) to chemotherapy alone. Gastrointestinal Tumor Study Group. J Natl Cancer Inst 80: 751-755. [Crossref]
- Klaassen DJ, MacIntyre JM, Catton GE, Engstrom PF, Moertel CG (1985) Treatment of locally unresectable cancer of the stomach and pancreas: a randomized comparison of 5-fluorouracil alone with radiation plus concurrent and maintenance 5-fluorouracil--an Eastern Cooperative Oncology Group study. J Clin Oncol 3: 373-378. [Crossref]
- Reyngold M, Parikh P, Crane CH (2019) Ablative radiation therapy for locally advanced pancreatic cancer: techniques and results. Radiat Oncol 14: 95. [Crossref]
- Teriaca MA, Loi M, Suker M, Eskens FALM, van Eijck CHJ, et al. (2021) A phase II study of stereotactic radiotherapy after FOLFIRINOX for locally advanced pancreatic cancer (LAPC-1 trial): Long-term outcome. Radiother Oncol 155: 232-236. [Crossref]
- Holyoake DLP, Robinson M, Silva M, Grose D, Mclntosch D, et al. (2021) SPARC, a phase-I trial of pre-operative, margin intensified, stereotactic body radiation therapy for pancreatic cancer. Radiother Oncol 155: 278-284. [Crossref]
- Cilla S, Ianiro A, Romano C, Deodato F, Macchia G, et al. (2020) Automated treatment planning as a dose escalation strategy for stereotactic radiation therapy in pancreatic cancer. J Appl Clin Med Phys 21: 48-57. [Crossref]
- Krishnan S, Chadha AS, Suh Y, Chen H, Rao A, et al. (2016) Focal Radiation Therapy Dose Escalation Improves Overall Survival in Locally Advanced Pancreatic Cancer Patients Receiving Induction Chemotherapy and Consolidative Chemoradiation. Int J Radiat Oncol Biol Phys 94: 755-765. [Crossref]
- Liermann J, Naumann P, Hommertgen A, Pohl M, Kieser M, et al. (2020) Carbon ion radiotherapy as definitive treatment in non-metastasized pancreatic cancer: study protocol of the prospective phase II PACK-study. BMC Cancer 20: 947. [Crossref]
- Cuneo KC, Morgan MA, Sahai V, Schipper MJ, Parsels LA, et al. (2019) Dose Escalation Trial of the Wee1 Inhibitor Adavosertib (AZD1775) in Combination With Gemcitabine and Radiation for Patients With Locally Advanced Pancreatic Cancer. J Clin Oncol 37: 2643-2650. [Crossref]
- Lin C, Verma V, Lazenby A, Ly QP, Berim LD, et al. (2019) Phase I/II Trial of Neoadjuvant Oregovomab-based Chemoimmunotherapy Followed by Stereotactic Body Radiotherapy and Nelfinavir For Locally Advanced Pancreatic Adenocarcinoma. Am J Clin Oncol 42: 755-760. [Crossref]
- Vainshtein JM, Schipper M, Zalupski MM, Lawrence TS, Abrams R, et al. (2013) Prognostic significance of carbohydrate antigen 19-9 in unresectable locally advanced pancreatic cancer treated with dose-escalated intensity modulated radiation therapy and concurrent full-dose gemcitabine: analysis of a prospective phase 1/2 dose escalation study. Int J Radiat Oncol Biol Phys 86: 96-101. [Crossref]
- Herman JM, Wild AT, Wang H, Tran PT, Chang KJ, et al. (2013) Randomized phase III multi-institutional study of TNFerade biologic with fluorouracil and radiotherapy for locally advanced pancreatic cancer: final results. J Clin Oncol 31: 886-894. [Crossref]
- Ikeda M, Ioka T, Ito Y, Yonemoto N, Nagase M, et al. (2013) A multicenter phase II trial of S-1 with concurrent radiation therapy for locally advanced pancreatic cancer. Int J Radiat Oncol Biol Phys 85: 163-169. [Crossref]
- Ben-Josef E, Schipper M, Francis IR, Hadley S, Ten-Haken R, et al. (2012) A phase I/II trial of intensity modulated radiation (IMRT) dose escalation with concurrent fixed-dose rate gemcitabine (FDR-G) in patients with unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 84: 1166-1171. [Crossref]
- Hunter KU, Feng FY, Griffith KA, Francis IR, Lawrence TS, et al. (2012) Radiation therapy with full-dose gemcitabine and oxaliplatin for unresectable pancreatic cancer. Int J Radiat Oncol Biol Phys 83: 921-926. [Crossref]
- Qing SW, Ju XP, Cao YS, Zhang HJ (2017) Dose escalation of Stereotactic Body Radiotherapy (SBRT) for locally advanced unresectable pancreatic cancer patients with CyberKnife: protocol of a phase I study. Radiat Oncol 12: 6. [Crossref]
- Shaib WL, Hawk N, Cassidy RJ, Zhang C, Brutcher E, et al. (2016) A Phase 1 Study of Stereotactic Body Radiation Therapy Dose Escalation for Borderline Resectable Pancreatic Cancer After Modified FOLFIRINOX (NCT01446458). Int J Radiat Oncol Biol Phys 96: 296-303. [Crossref]
- Wild AT, Herman JM, Dholakia AS, Moningi S, Lu Y, et al. (2016) Lymphocyte-Sparing Effect of Stereotactic Body Radiation Therapy in Patients With Unresectable Pancreatic Cancer. Int J Radiat Oncol Biol Phys 94: 571-579. [Crossref]
- Herman JM, Chang DT, Goodman KA, Dholakia AS, Raman SP, et al. (2015) Phase 2 multi-institutional trial evaluating gemcitabine and stereotactic body radiotherapy for patients with locally advanced unresectable pancreatic adenocarcinoma. Cancer 121: 1128-1137. [Crossref]
- Combs SE, Habermehl D, Kieser M, Dreher C, Werner J, et al. (2013) Phase I study evaluating the treatment of patients with locally advanced pancreatic cancer with carbon ion radiotherapy: the PHOENIX-01 trial. BMC Cancer 13: 419. [Crossref]
- Lin C, Verma V, Ly QP, Lazenby A, Sasson A, et al. (2019) Phase I trial of concurrent stereotactic body radiotherapy and nelfinavir for locally advanced borderline or unresectable pancreatic adenocarcinoma. Radiother Oncol 132: 55-62. [Crossref]
- Heerkens HD, van Vulpen M, Erickson B, Reerink O, Intven MP, et al. (2018) MRI guided stereotactic radiotherapy for locally advanced pancreatic cancer. Br J Radiol 91: 20170563 [Crossref]
- Comito T, Cozzi L, Clerici E, Franzese C, Tozzi A, et al. (2017) Can Stereotactic Body Radiation Therapy Be a Viable and Efficient Therapeutic Option for Unresectable Locally Advanced Pancreatic Adenocarcinoma? Results of a Phase 2 Study. Technol Cancer Res Treat 16: 295-301. [Crossref]





