Abstract
Acellular dermal matrix (ADM) is commonly utilized in breast reconstructive surgery. The primary objective of this study is to identify the typical imaging features of ADM on different modalities and correlate this information with the known surgical history and pathology to help achieve the correct diagnosis, thereby avoiding unnecessary work-up. We present a pictorial review of cases from our institution and characterize the imaging findings of ADM in patients with breast cancer who underwent post-mastectomy breast reconstruction. The common clinical presentation of ADM includes palpable mass, pain or tenderness. Ultrasound findings of ADM include circumscribed or irregular homogeneous isoechoic or hypoechoic masses with circumscribed or indistinct margins. On magnetic resonance imaging (MRI), ADM usually presents as a non-enhancing mass. The differential diagnosis of ADM makes post-surgical imaging difficult as it includes fat necrosis, recurrence of malignant disease, and a host of complications including seroma, hematoma, and infection. Our cases include one biopsy proven case of ADM and one case which demonstrated both recurrent disease and ADM. Understanding the characteristic imaging features of ADM in the reconstructed breast, the differential diagnosis, and correlation with plastic surgery history will help improve diagnostic accuracy in the post-operative setting.
Keywords
acellular dermal matrix, breast cancer, reconstruction, implants, breast MRI, breast ultrasound
Introduction
Acellular dermal matrix (ADM) is a biologic scaffold with intact basement membrane and cellular matrix from which antigenic components have been removed and thus this may be integrated into the host tissue via revascularization and tissue remodeling[1-3]ADM is now commonly utilized in breast reconstructive surgery including in breast cancer patients and was first described by Brueing and Warren in 2005 [4].Although ADM may have human cadaver, fetal bovine or porcine origin, one of the most commonly used, AlloDerm (LifeCell Corp., Branchburg, N.J.), is of human origin[2,3,5].Specifically, AlloDerm may be used for post-lumpectomy filler, post-mastectomy 2-stage reconstruction or immediate breast reconstruction. ADM has facilitated direct to implant breast reconstruction which eliminates the need for tissue expanders, and may promote faster recovery [1,6,7].
Imaging in the post-operative state serves the purpose of identifying complications or ruling out recurrent breast cancer in the setting of symptoms such as pain or a new palpable finding [5].The presence of ADM confounds post-operative imaging and makes diagnosis challenging given its variable presentation and similarity to the appearance of complications or recurrence, particularly when it presents as a mass. The common differential diagnoses include ADM, fat necrosis and recurrent malignancy as well as abscess, seroma, and hematoma. The first-line imaging modality is usually targeted ultrasound in these patients. A mammogram is often obtained if fat necrosis is high on the differential diagnosis in order to see calcifications or fat-density masses [8].
In our series of cases, we demonstrate typical findings of ADM,ADM findings different from fat necrosis, and an uncommon case of invasive breast cancer arising adjacent to ADM.
Cases
Case 1
48-year-old female with history of left breast ER+, PR+ ductal carcinoma in situ (DCIS) intermediate to high nuclear grade with necrosis, solid and comedo types who is status post bilateral skin-sparing mastectomy and two-stage reconstruction with placement of ADM and bilateral prepectoral silicone gel implants. Two years after surgery, the patient presented with a new “eraser-size” palpable finding for 2 months and underwent limited left breast ultrasound (Figure 1).
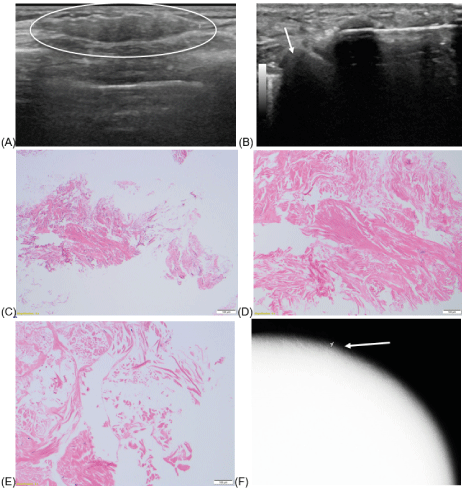
Figure 1:
Left breast ultrasound, greyscale transverse (A) image at palpable finding demonstrate an oval parallel isoechoic mass with circumscribed margins measuring 9 x 4 x 27 mm seen in the axillary tail of the left breast (circle). No vascularity is associated with the lesion. This mass is located at the edge of the implant and adjacent implant is partially visualized next to the mass (arrow) when biopsy was performed (B).This mass was biopsied due to its “recent growth” reported by the patient. Pathology stated that based on the clinical history, the presence of partially acellular collagen tissue may represent acellular dermal matrix (C,D,E).
Figure 1C. Low power view showing cellular fibrous tissue (right lower corner) and detached thick bundles of partially cellular collagen (H&E 4X).
Figure 1D. Thick collagen bundles with scattered stromal nuclei. No inflammation or neovascularization (H&E 10X)
Figure 1E. Acellular collagen in thick interlacing fascicles. (H&E 20X).
Figure 1F. Post-biopsy left MLO mammogram shows a clip (arrow) next to the implant.
Case 2
47-year-old female with history of right breast ER+, PR+ DCIS arising in association with a sclerosing papilloma status post bilateral skin-sparing total mastectomy and two-stage reconstruction with placement of ADM and bilateral prepectoral silicone gel implants. Two years after surgery, the patient presented with bilateral palpable findings and underwent targeted ultrasound as well as MRI. Patient subsequently underwent revision of right breast reconstruction (Figure 2).
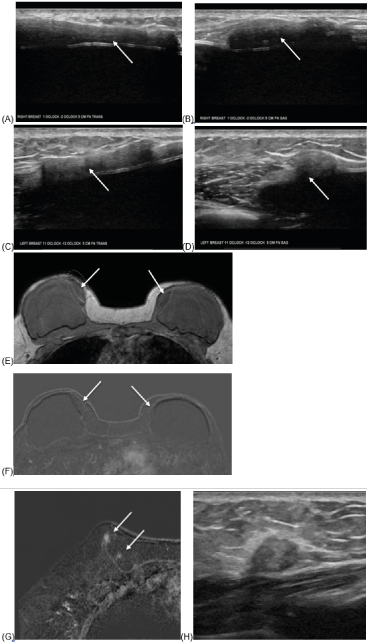
Figure 2:
Left and right breast ultrasound, greyscale transverse (A,C) and sagittal (B, D) images at palpable findings demonstrate similar appearing hypo/isoechoic sheet-like structures along the medial aspects of both implants, at 1-2:00, 5 cm from the nipple in the right breast (A,B) measuring 48 x 7 x 28 mm and in the left breast from 11-12:00, 5 cm from the nipple (C,D) measuring 32 x 5 x 11 mm (arrows) . There is no internal vascularity. Differential diagnoses include fat from reported fat grafts, extracapsular silicone, or other postsurgical change. MRI recommended.
Subsequent MRI with representative axial T1 non-fat saturated image (E) demonstrates isointense oval mass in the upper inner quadrant of the bilateral breasts (arrows). Subtraction post contrast T1 image (F) shows no enhancement in these areas. (G) There is also a tiny foci of enhancement in the right upper inner breast, most likely fat necrosis (arrows). Ultrasound of corresponding area showed heterogeneously isoechoic mass with surrounding hyperechogenicity (H). Biopsy of this mass showed fat necrosis. Repeat MRIs at 6 months and 1 year (no images included) demonstrated stable medial ADM and stable to decreasing foci of enhancement at the area of the biopsy-proven fat necrosis.
Case 3
41-year-old female with history of left breast ER+, PR+, HER2- invasive ductal carcinoma (IDC) status post bilateral total mastectomy and two-stage reconstruction with placement of ADM and prepectoral silicone gel implants. Six months after surgery, the patient presented with palpable findings in the bilateral breasts and underwent targeted ultrasound (Figure 3).
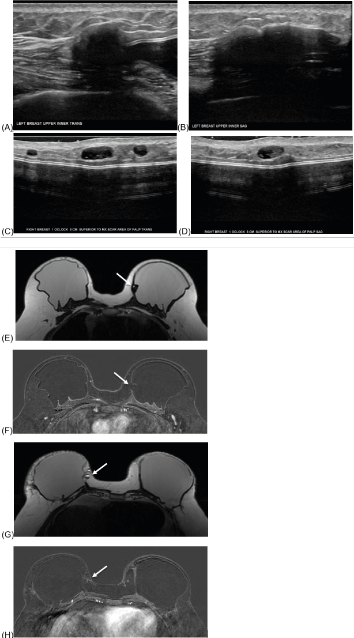
Figure 3:
Left breast ultrasound, greyscale transverse (A) and sagittal (B) images at palpable finding demonstrates a hypoechoic mass likely representing ADM or fat necrosis.
Right breast ultrasound, greyscale transverse (C) and sagittal (D) images at palpable finding demonstrates multiple similar oval circumscribed cystic masses consistent with oil cysts.
Subsequent MRI with representative axial post-contrast T1 image shows a T1 hypointense, non-enhancing area along the upper inner aspect of the left implant which correlates with sonographic finding and is favored to represent ADM (E, F, arrows). Representative axial images of the right upper inner breast (G, H, arrows) demonstrate medial right breast fat necrosis (arrows).
Case 4
41-year-old transgender female who tested positive for BRCA gene and underwent bilateral total mastectomy and two stage reconstruction with placement of ADM and prepectoral silicone gel implants. Afterwards, the patient presented with tenderness and firmness on the lateral aspect of the right breast and underwent MRI (Figure 4).
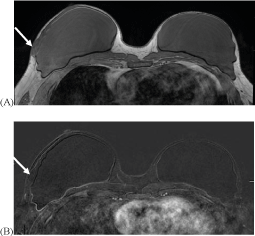
Figure 4:
MRI with representative axial non-contrast non-fat saturated T1 image demonstrates no residual glandular tissue. There is an isointense oval, circumscribed mass along the lateral aspect of the right reconstructed breast at the level of the symptoms which measures approximately 2.6 x 1 cm (A, arrow). This did not enhance and was consistent with ADM, confirmed with the surgical record (B, arrow).
Case 5
40-year-old female with history of DCIS of the left breast status post skin sparing mastectomy with immediate prepectoral reconstruction with ADM and silicone implant (Figures 5,6).
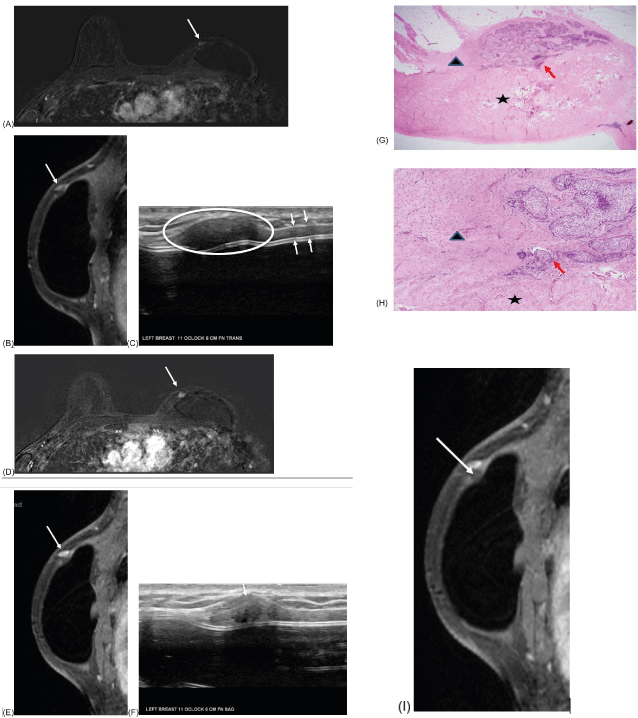
Figure 5:
Surveillance axial and sagittal MRI images 12 months after lumpectomy showed a focal nonmass enhancement in the upper inner left breast, measuring approximately 4 x 9 x 3 mm (A, B, arrows). Enhancement pattern showed plateau kinetics (no image). This was thought to most likely represent fat necrosis.
Subsequent ultrasound showed a circumscribed, oval hypoechoic mass without vascularity, measuring 16 x 5 x 7 mm in the left breast at 11 o'clock located 6 centimeters from the nipple (circles), most consistent with ADM (C). Sheet-like nonpalpable ADM is also demonstrated next to the implant (C, arrows). Six month follow-up was recommended. Follow-up ultrasound showed stable findings (not shown).
Surveillance axial and sagittal MRI images in 12 months showed the enhancing mass increased in size, measuring approximately 7 x 11 x 4 mm (D, E, arrows). Subsequent ultrasound showed minimal focal bulging area within the previously seen stable oval mass (F, arrow). Although the ultrasound findings are not significantly different, biopsy was performed based on the MR findings. Pathology showed invasive ductal carcinoma with adjacent ADM (G, H). Retrospectively, there is associated nonenhancing mass below the enhancing mass, which is likely representing adjacent ADM (I, arrows).
Figure 5 G, H: H/E stain to show relationship of invasive ductal carcinoma (IDC) with acellular dermal matrix (ADM). IDC (by red arrow) is located in patient’s fibroadipose tissue (by black triangle). IDC is directly continuous but not invade into ADM (by black star). ADM is characterized by disorganized pink collagen fibers with scant fibroblasts and neovascularization. In addition, ADM also gradually blends into adjacent benign skeletal muscle (photo not shown).

Figure 6:
Photograph shows the appearance of total coverage of ADM over an implant.
Discussion
ADM is usually used to cover the implant to improve stabilization and to decrease future capsular contracture[1-3].For pre-pectoral implants, ADM covers the entire implant (Figure 6). For retro-pectoral implants, ADM covers the lower outer part of the implant where it is not covered by the pectoralis muscle [2,4,6].ADM can cover only the anterior aspect of the implant in some cases (“partial coverage”). Brueing originally described a sling technique to create this coverage but other methods of ADM insertion have also been described in the literature including rolled and diced[7,9].ADM is also utilized in pre-pectoral breast reconstruction in nipple-skin sparing mastectomies [6].While ADM usage can be beneficial in regards to cosmetic outcome, decreased contracture rate, decreased post-operative pain, and decreased operative time, the complication rate is not lower than in non-ADM reconstructions [1]. Possible other complications from ADM include infection, hematoma, seroma, and fat necrosis.
In total coverage, ADM should evenly cover the entire implant, but various degrees of folding can happen during surgery, and sutures can be put at the edges of the implant to enhance stabilization. In partial coverage, sutures can be put at the junction of the implant-pectoralis muscle. After implantation, ADM can be vascularized and integrated to the surrounding tissue. If not fully integrated, it may make a mass-like structure. Folding and suture sites may create mass-like structures as well. The usual thickness of ADM is 1-1.5 mm. This may become thicker after the implantation in vivo over time. ADM may show different enhancement on imaging studies, according to its different stages of vascularization.
At our institution, most patients underwent skin-sparing or nipple sparing total mastectomies and two-stage reconstruction with placement of tissue expanders with ADM and later replacement of tissue expanders with prepectoral silicone implants. The brand of ADM utilized was not specified. Patients presented with symptoms of pain or palpable finding approximately 6 months to 3 years after their last surgery. The initial imaging exam was almost always ultrasound due to the post-mastectomy status of these patients. Sonographic findings were hypoechoic or isoechoic, nonvascular masses. The normal shape of ADM is either a sheet-like or an oval mass especially when it presents as a palpable mass.
There was overlap of sonographic findings of ADM and fat necrosis which can be clarified by MRI. In our cases, non-enhancing masses with no internal fat signal were suggestive of ADM rather than fat necrosis.
The literature describes a spectrum of imaging findings for ADM on various modalities. AlloDerm appears as an equal to high density mass on mammography without obscuration of calcifications[7,8,10].On ultrasound, AlloDerm has been described as isoechoic to hypoechoic masses with smooth margins and variable internal color flow[8,10,11].On MRI, AlloDerm was isointense to glandular tissue on non-contrast fat-saturated T1-weighted images, hyperintense on fat-saturated T2-weighted images, and had variable post-contrast enhancement[7,8,10,12].This variability is attributed to the different stages and extent of neovascularization and incorporation of the ADM into the host [11].Specifically, regarding MRI enhancement, Lee et al [8]stated that initially there is minimal to any enhancement of the ADM, but this transitions to similar enhancement to background after the matrix is completely incorporated into the host [8].All of our cases showed nonenhancing masses on MRI.
Kim et al. recently created a classification system to grade the sonographic findings of ADM and correlate them to their respective histopathologic states in the early postoperative period, up to 1 year after surgery [13].Type 1 findings demonstrated a focal thickening with decreased echogenicity which correlated with chronic inflammatory infiltrate. Type 2 included diffusely increased echogenicity which correlated with dense collagen bundles. Type 3 represented echogenic spots within the ADM which correlated with empty spaces inside the ADM. Calcifications were not observed [13].
According to Kim et al [10]abbreviated MRI surveillance could be useful for detecting ipsilateral local tumor recurrence and avoiding ADM-related diagnostic dilemma and delay. Their study found that the ipsilateral local tumor recurrence rate was 2.5% in patients with ADM, which is comparable to prior studies. They found that 62.5% of cancers in their study would have been missed with mammography alone, and 37.5% (3 of 8) might have been missed with a combination of mammogram and ultrasound. Interestingly, 66.7% (2 of 3) of cancers which were only seen on abbreviated MRI were located along the margin of the excision cavity and obscured by ADM on mammogram and ultrasound [10].In their study, the sensitivity and specificity of mammogram, ultrasound, both mammogram and ultrasound, and abbreviated MRI in detection of ipsilateral local tumor recurrence in patients with ADM were 37.5%, 50%, 62.5%, and 100% and 99.7%, 98.4%, 98.1%, and 97.8%, respectively. Although the number of recurrent cancers in this study was small (8 cases), the results show that abbreviated MRI has the advantage in detection of local recurrence over mammography and ultrasound.
Conclusion
This case series presents multiple cases of patients who have undergone mastectomies and two-stage reconstruction, including implants with ADM. The imaging findings of pure ADM in our cases are mostly specific on ultrasound and different from fat necrosis. MRI is an accurate imaging modality to differentiate ADM from fat necrosis or nearby malignancy. Understanding the characteristic imaging features of ADM in the reconstructed breast as well as the differential diagnoses will help avoid unnecessary work-up and improve patient care.
References
- Bertozzi N, Pesce M, Santi P, Raposio E (2017) One-Stage Immediate Breast Reconstruction: A Concise Review. BioMed Res Int 2017: 6486859. [Crossref]
- Salzberg CA (2006) Nonexpansive immediate breast reconstruction using human acellular tissue matrix graft (AlloDerm). Ann PlastSurg 57: 1-5. [Crossref]
- Jansen LA, Macadam SA (2011) The Use of AlloDerm in Postmastectomy Alloplastic Breast Reconstruction: Part I. A Systematic Review.PlastReconstrSurg 127: 2232-2244. [Crossref]
- Breuing KH, Warren SM (2005) Immediate Bilateral Breast Reconstruction With Implants and Inferolateral AlloDerm Slings. Ann PlastSurg55:232-239. [Crossref]
- Lee C, Bobr A, Torres-Mora J (2017) Radiologic-Pathologic Correlation: Acellular Dermal Matrix (Alloderm[sup][R]) Used in Breast Reconstructive Surgery. J Clin Imaging Sci7:13-13. [Crossref]
- Cuomo R (2020)Submuscular and Pre-pectoral ADM Assisted Immediate Breast Reconstruction: A Literature Review. Medicina (Kaunas)56:256. [Crossref]
- Cao HST, Tokin C, Konop J, Ojeda-Fournier H, Chao J, et al. (2010) A Preliminary Report on the Clinical Experience with AlloDerm in Breast Reconstruction and its Radiologic Appearance. Am Surg76:1123-1126. [Crossref]
- Lee CU, Clapp AJ, Jacobson SR (2014) Imaging Features of AlloDerm(®) Used in Postmastectomy Breast Reconstructions. J Clin Imaging Sci4:19. [Crossref]
- Gwak H, Jeon YW, Lim ST, Park SY, Suh YJ (2020) Volume replacement with diced acellular dermal matrix in oncoplastic breast-conserving surgery: a prospective single-center experience. World J SurgOncol18:60. [Crossref]
- Kim MY, Suh YJ, An YY (2021) Imaging surveillance for the detection of ipsilateral local tumor recurrence in patients who underwent oncoplastic breast-conserving surgery with acellular dermal matrix: abbreviated MRI versus conventional mammography and ultrasonography. World J SurgOncol19:290. [Crossref]
- Seon Kim Y (2016) Ultrasonography Findings of AlloDerm® Used in Postmastectomy Alloplastic Breast Reconstruction: A Case Report and Literature Review. Iran J Radiol13:e38252. [Crossref]
- Lee CB, Kim YS, Lee SE (2022) Imaging features of volume replacement using an acellular dermal matrix in oncoplastic breast conserving surgery: A case report. Radiol Case Rep17:2146-2149. [Crossref]
- Kim YS, Lee WS, Park BY, Choi M, Lee JH, et al. (2022) Abnormal Ultrasonographic Findings of Acellular Dermal Matrix in Implant-Based Breast Reconstruction: Correlations with Histopathology. J Clin Med11:1057. [Crossref]






