The consumption of dietary supplements advertised as slimming agents is increasing with the prevalence of obesity. However, several of these supplements are adulterated with pharmacologically active substances or with ingredients lacking any demonstration of their efficiency. Alongside the phytochemicals endowed with lipolytic properties such as caffeine or synephrine, the alkaloid hordenine, naturally present in germinated barley and in other plants, belongs to such ingredients added to dietary supplements advertised to improve lipid mobilization in consumers. Since the lipolytic properties of hordenine were not documented so far, we aimed to verify the in vitro rapid effects of hordenine on rodent adipocyte lipolytic and antilipolytic responses. It was found that different doses of hordenine do not activate or inhibit lipolysis in mouse fat cells, which were responsive to the stimulatory (isoprenaline, forskolin) or inhibitory (insulin) control of triglyceride breakdown. Moreover, in human adipose tissue, hordenine interacted with monoamine oxidase (MAO) and competed for the oxidation of radiolabeled tyramine while it was not a substrate, or an inhibitor of the semicarbazide-sensitive amine oxidase involved in the degradation of benzylamine. Our results indicated that hordenine does not reproduce the already documented antilipolytic effects of tyramine or the lipolytic action of synephrine, two of its closely related naturally occurring phenethylamines.
Adipocytes; Lipolysis; Monoamine oxidase; Semicarbazide-sensitive amine oxidase; Obesity; Plant alkaloids; N-Methyltyramine
Hordenine is a naturally occurring molecule belonging to the families of phenethylamines. It is also commonly called dimethyltyramine and is a close derivative of N-methyltyramine. Hordenine is present in various edible plants, and especially in cereals, with barley as the most important example, being at the origin of the name hordenine (Hordenum species, exhibiting levels up to 2mg/g upon germination). Since this alkaloid is extracted from barley malt during beer production, it is commonly ingested by millions of consumers in the low milligram range [1]. Hordenine is also found in diverse species of cactus and algae, and in Citrus fruits [2]. Most importantly, hordenine is present or added in nutritional supplements advertised to promote weight loss [2-4] while the scientific literature lacks clear demonstration of the lipid mobilizing or thermogenic properties of this phytochemical.
Synephrine, the predominant adrenergic protoalkaloid found in the peel and fruit of bitter orange (Citrus aurantium) is reputed to increase energy expenditure and to be safe [5], while it has been reported to activate lipolysis from fat stores [6,7]. However, the use of related protoalkaloids (tyramine, N-methyltyramine, octopamine, and hordenine) remains questionable [2,3]. Theses alkaloids are present together with synephrine in Citrus fruits, but their lipolytic activity is far from being convincing. Even more, several supplements advertised for weight loss (containing or not Citrus extracts) are adulterated with caffeine or other pharmacologically active substances, which are out of the scope of the present study, [4,8]. Since the phenethylamine alkaloid hordenine belongs to such ingredients endowed with anti-obesity properties and added to dietary supplements for improving lipid mobilization in consumers [8], the objective of this work was to test hordenine on fat cell lipolysis. Indeed, the triglycerides stored in fat cells (in an excessive manner when energy supply is greater than energy expenditure) represent a fuel store that can be recruited upon demand under the form of glycerol and FFA, the products of adipocyte lipolysis, which are released in the blood stream and used as substrates by other organs. Whether hordenine activates this process in a short-term manner needs to be established.
Consequently, the dose-dependent influence of hordenine on the glycerol and free fatty acid (FFA) release was compared to that of recognized lipolytic agents: isoprenaline and forskolin (β-adrenergic agonist and adenylyl cyclase activator, respectively). Besides being supposed to activate lipolysis in fat cells, hordenine is also a tyramine derivative. Since we have recently reported that millimolar doses of tyramine activate glucose uptake and inhibit lipolysis in rodent adipocytes [9,10], and are clearly antilipolytic in human fat cells [11], our planned investigations also verified whether hordenine inhibits lipolysis. This working hypothesis was even reinforced by another evidence: the catecholamines themselves (adrenaline, noradrenaline), which belong to the natural messengers regulating lipid mobilization, are able to activate and also to inhibit lipolytic activity, via the respective stimulation of β-adrenergic receptors (β-AR) and of a2-adrenergic receptors (a2-AR). The β-AR stimulation increases cyclic adenosine monophosphate (cAMP) production in fat cells while that of a2-AR decrease it. The response of fat cells is driven thereby by their adrenoceptor equipment [12]. In addition, Sommer and coworkers clearly showed that hordenine dose-dependently inhibited cAMP production in forskolin-stimulated cultured in human embryonic kidney cells [1]. Although this finding was due to a biased agonism at dopaminergic D2-receptors, it reinforced our interest in investigating both putative lipolytic and antilipolytic effects of hordenine.
Catecholamines and dopamine are substrates of MAO-A and MAO-B, which support the termination of action of these neurotransmitters [13]. In view of its chemical structure, hordenine might also interact with such mitochondrial enzymes as well as with other amine oxidases highly expressed in adipocytes. Since benzylamine and methylamine contribute to the regulation of lipolytic and lipogenic activities of fat cells via their oxidation by amine oxidases different from MAO, namely semicarbazide-sensitive amine oxidases (SSAO) [14,15], a putative interaction of hordenine with these membrane-bound enzymes was included in the following study.
Differently from the exploration of lipolysis pathway that requires adipose cell integrity, the assay of amine oxidase activity can be performed on acellular biological resource, such as purified enzymes or crude homogenates. We take the opportunity of available frozen pieces of human adipose tissue (AT) to investigate a putative interaction between hordenine and the human forms of MAO and SSAO.
It was out of the scope of the present work to define the mean daily intake of hordenine by consumers since this aspect has been already analyzed [16]. On the contrary, this study will focus attention on the short-term effects of increasing doses of hordenine in rodent adipocytes and in human AT. Since hordenine has already been tested at 100 and 500 µM on human melanocytes [17], we used for the following study, a dose range comprised between 1 µM and 1 mM. At these doses, no substantial direct regulatory effect was found on triglyceride breakdown in mouse adipocytes, while an interaction with human MAO, but not SSAO, was confirmed.
Chemicals
Hordenine, tyramine hydrochloride, (−)-isoprenaline hydrochloride, hydrogen peroxide (H2O2), semicarbazide, insulin and albumin (of bovine origin), and other reagents were obtained from Sigma-Aldrich (Saint Quentin Fallavier, France), as it was also the case for [14C]-tyramine. The compounds Ro 41-1049 (N-(2-Aminoethyl)-5-(3-fluorophenyl)-4-thiazolecarboxamide hydrochloride) and Ro 16-6491 (N-(2-Aminoethyl)-4-chlorobenzamide) were obtained from Roche (Neuilly-sur-Seine, France) and served as selective MAO-A and MAO-B blockers, respectively. DMSO was used at 50% v/v to dissolve caffeine and hordenine for stock solutions kept at 6°C. The chemical structures of hordenine and N-methyltyramine are not presented in this report since they are given in the first figure of the freely accessible work of Sommer and coworkers [1].
Animals
Twenty C57J6 mice of both genders were obtained from (Charles River, L’Arbresle, France) and grown at CREFRE (Centre Régional d’Exploration Fonctionnelle et Ressources Expérimentales, Toulouse, France) and kept in plastic boxes containing a maximum of four animals per cage. They were fed a standard rodent chow and received water ad libitum under controlled environment (12:12 h light-dark cycle and temperature of 22 ± 2 °C). All animal procedures complied with the principles established by the Institut National de la Santé et de la Recherche Médicale (INSERM, France) according to the Protocol Permission Number 12-1048-03-15 (on the 20/03/2012) and were approved by the local Ethics Committee. Adipose tissue was removed from subcutaneous or visceral, including perigonadic, anatomical locations as already detailed [18], and pooled for the subsequent preparation of freshly isolated adipocytes.
Adipose cell functional explorations
Minced pieces of adipose depots were digested by 1 mg/mL collagenase type II for approximately 45 min under agitation in Krebs-Ringer salt solution pH 7.5 containing 15 mM sodium bicarbonate, 10 mM HEPES and 3.5% of fat-depleted albumin. The isolation, and washing steps, as well as the distribution of fat cell suspension into assay wells were performed as previously described [19]. Lipolytic activity was assessed by determining the glycerol released in the medium after 90-min incubation of adipocytes according to [19]. Free fatty acid release was also another index measured in parallel to study the influence of tested agents on triglyceride breakdown, as in [20].
Human adipose tissue samples
The adipose samples were obtained from six women undergoing abdominal surgery at the Rangueil hospital, Toulouse (France) with their informed consent. Their mean body mass (BMI) was 26.1 ± 1.8 kg/m2. Samples of abdominal subcutaneous AT were immediately frozen in liquid nitrogen and then stored at -80°C, until their use. AT samples were thawed at room temperature and homogenized in 200 mM phosphate buffer (pH 7.5) as already described [21] and just before the different types of amine oxidase activity assays, using either radiometric or fluorometric methods.
Assessment of amine oxidation
The oxidation of 0.5 mM [14C]-tyramine was performed, as described in [22], using human AT homogenates as a biological source of amine oxidases. Frozen pieces of subcutaneous abdominal adipose depots were thawed and prepared as described above. Amine oxidation was then determined on 30-min incubation at 37 °C, as already described [23]. As already described [24], the extraction and the counting of aldehydic products was performed in ethyle acetate/toluene and in liquid scintillation, respectively. The reference SSAO inhibitor (1 mM semicarbazide) impaired less than 5 % of the production of tyramine-related radioactive aldehydes, while it almost totally obliterated the benzylamine-induced hydrogen peroxide production, which was determined according to an Amplex Red-based fluorometric method, as in [25]. Protein quantification was performed using DC Protein Assay kit (BioRad, Hercules, CA, USA), then amine oxidase activity expressed as nmoles of oxidation product/mg protein/min.
Statistical analysis
Results are presented as mean ± standard error of the mean (SEM). Statistical analysis for comparisons between treated and respective control used ANOVA followed by post hoc tests. NS: non significantly different from respective control.
Lipolytic activity
The first tests of the direct influence of hordenine on lipolysis were performed on 90-min incubation with adipocytes isolated from seven-month-old mice. The lipolytic agents of reference were the β-adrenergic agonist isoprenaline and the direct activator of adenylyl cyclase forskolin. Both lipolytic agents stimulated glycerol and free fatty acid (FFA) release. The maximal lipolysis was reached with 10 µM isoprenaline, which was set at 100% (Figure 1). Adrenocorticotropic hormone (ACTH) was also significantly lipolytic at 1 µM, validating the functionality of the tested adipocyte preparations. In these conditions, hordenine was inactive on glycerol release and on FFA release when tested from 1 µM to 1 mM (Figure 1).
Figure 1. Comparative study of the effects of hordenine and other lipolytic agents in mouse adipocytes.
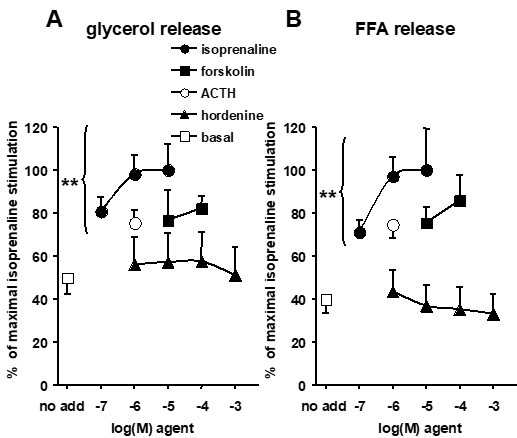
Mouse adipocytes were incubated for 90 min without (basal, open square) or with the indicated concentrations of hordenine (closed triangles). The lipolytic agents of reference, isoprenaline (closed circles), forskolin (closed squares), and adrenocorticotropic hormone (ACTH, open circle) were also added at the beginning of the incubation. The release of A) Glycerol and B) free fatty acids (FFA) ito the incubation medium was measured at the end of the incubation. Mean ± SEM of 8 adipocyte preparations. Different from basal lipolysis at: ** p < 0.01.
However, the relatively elevated basal values of lipolysis, together with the relatively modest stimulation by isoprenaline found in these adipocytes (2- to 3-fold activation), prompted us to verify whether hordenine could be more active in more responsive adipocytes from younger mice. Further analyses were therefore performed on six additional adipocyte preparations obtained from two-month-old mice.
In this second set of experiments, fat cells were more responsive to isoprenaline since they activated their basal glycerol and FFA release by seven-to ten-fold (Figure 2). Hordenine at 100 µM did not alter lipolytic activity, neither in basal nor in stimulated conditions.
Figure 2. Lack of lipolytic and antilipolytic effect of hordenine in mouse adipocytes.
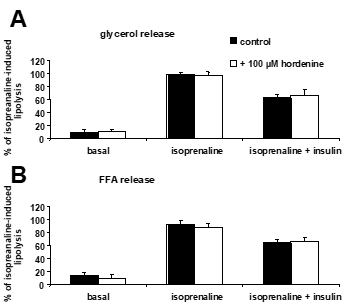
The release of glycerol (A) and free fatty acids (B) was measured at the end of 90 min incubation and results were expressed as percentage of 0.1 µM isoprenaline-dependent lipolysis, in the absence of any agent or vehicle. No significant difference was found between without (control: 1 % DMSO vehicle, closed columns) and with 100 µM hordenine (open columns) for each condition tested. Mean ± SEM of 8 experiments.
Although hordenine was apparently devoid of any effect, it was studied whether the amine could influence the antilipolytic effect of insulin. The presence of 0.1 µM insulin inhibited by approximately one-third of the lipolytic effect of 0.1 µM isoprenaline, and this remained unchanged in the presence of 100 µM hordenine (Figure 2). Hordenine was therefore not modifying the antilipolytic response to insulin.
Taken together, these data indicated that hordenine could not mimic or impair the activatory action of isoprenaline, and that it could not mimic or impair the inhibitory action of insulin. Thus, hordenine cannot be considered as capable to directly regulate triglyceride breakdown in mouse adipocytes that are responsive to other lipolytic and antilipolytic agents.
Apparently, hordenine was not interacting in a functional manner with membrane receptors that are stimulated by other biogenic amines (e.g. catecholamines), and which positively or negatively regulate triglyceride breakdown in adipocytes. However, since being a biogenic amine, hordenine can interact with other steps involved in aminergic systems such as enzymes involved in neurotransmitter clearance.
Monoamine oxidase activity
Adipocytes are known to express MAO and SSAO, two types of amine oxidases that degrade partially overlapping panels of soluble amines. Because phenethylamines, to which belongs hordenine, are MAO substrates, and because MAOs are involved in the regulation of energy balance, further investigation on the elusive interplay between MAO and hordenine appeared highly relevant. To this aim, the oxidation of [14C]-radiolabelled tyramine was measured in the absence and the presence of hordenine and related metabolites. The oxidation of 0.5 mM [14C]-tyramine by homogenates of human subcutaneous AT was dose-dependently abolished by the MAO-A inhibitor Ro 411049 (Figure 3). In contrast, the MAO-B inhibitor Ro 166491 was less potent, in agreement with the predominance of MAO-A reported in human AT [26]. Cold tyramine also competed for the oxidation of radiolabelled tyramine: it limited the detection of radiolabeled products of oxidation, as a classical effect of isotopic dilution. Interestingly, hordenine also competed for tyramine oxidation, suggesting that it could act as substrate for human MAO (Figure 3). As expected, the SSAO blocking agent semicarbazide at 1 mM was unable to impair [14C]-tyramine oxidation. This confirmed that tyramine is mostly oxidized by MAO in human AT and not by copper-containing amine oxidases such as SSAO. Caffeine was also tested in parallel conditions since this lipolytic alkaloid has been described to inhibit MAO [27] and SSAO [21,28]. Caffeine was able to partially impair the oxidation of radiolabeled tyramine since at 1 mM it produced 38 ± 5 % inhibition (n = 6, not shown).
Figure 3. Influence of hordenine and of reference MAO-inhibitors on tyramine oxidation by human adipose tissue.
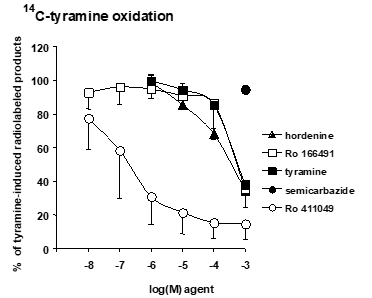
The oxidation of 0.5 mM [14C]-tyramine by human AT homogenates was set at 100 % in the absence of any added agent, while background was set at 0 %. The production of radioactive aldehydes derived from [14C]-tyramine oxidation was dose-dependently inhibited by hordenine (closed triangles) and by the selective MAO-A inhibitor (Ro-411049, open circles), the MAO-B inhibitor Ro-166491 (open squares), or even by cold tyramine (closed squares). Tyramine oxidation was not inhibited by 1 mM semicarbazide (closed circles). Each point is the mean ± SEM from 4 to 6 preparations from overweight subjects.
Indeed, in these experiments, it was difficult to distinguish the inhibition curves of inhibitory agents from those of competitive substrates: for instance, the curves of Ro 166491 and tyramine were almost superimposed in Figure 3. In other words, it was unclear whether hordenine actually competed for tyramine oxidation by being oxidized or whether it was impairing MAO activity without undergoing oxidative deamination. If hordenine is really oxidized by adipose cells, it has to produce ammonia, hydrogen peroxide and the corresponding aromatic aldehyde, which are all end-products of catabolism by amine oxidases, irrespective of the nature of the substrate or the enzyme [13]. It was therefore mandatory to verify whether hordenine promoted hydrogen peroxide release in human AT homogenates like other well-recognized amine oxidase substrates.
Hydrogen peroxide production
It was measured how the human AT preparations were generating hydrogen peroxide (H2O2) in response to hordenine in comparison with other MAO or SSAO substrates. When benzylamine was incubated at 1 mM for 30 min, it increased by 7.4 ± 1.0 fold the spontaneous H2O2 release of human AT homogenates (n = 6, P<0.001, not shown), as already documented [14]. 1 mM hordenine induced only 10.3 % of this release, while in the same condition’s tyramine increased baseline by 2.1 ± 0.3 fold (n = 6, P<0.01, not shown). This suggested that hordenine was not readily oxidized by any of the amine oxidases present in human AT. As expected, the benzylamine-induced H2O2 release was blocked by semicarbazide, therefore SSAO-mediated, while the tyramine action was prevented by pargyline, therefore MAO-dependent. By contrast, no significant effect of inhibitors could be detected on the weak hordenine-induced H2O2 production (not shown).
Semicarbazide-sensitive amine oxidase activity
It was then investigated whether hordenine and benzylamine could add their capacity to be oxidized by human AT. Figure 4 shows that the combination of hordenine with 0.5 mM benzylamine, did not result in an additive H2O2 production, as it was the case with 0.1 mM methylamine, another SSAO substrate. Moreover, hordenine did not inhibit benzylamine oxidation as did increasing doses of semicarbazide and b-aminopropionitrile, two blockers of copper-containing amine oxidases (Figure 4). Again, caffeine partially impaired benzylamine oxidation since, at 1 mM, it inhibited 20 ± 2 % of the benzylamine-dependent H2O2 production (n = 6, not shown).
Figure 4. Lack of influence of hordenine on benzylamine oxidation by human adipose tissue.

The oxidation of 0.5 mM benzylamine by human AT homogenates induced hydrogen peroxide release that was set at 100 % in the absence of any added agent, while baseline was set at 0 %. The hydrogen peroxide release was dose-dependently inhibited either by the reference SSAO inhibitor semicarbazide (closed circles), b-aminopropionitrile (b-APN, closed squares), and less affected by the MAO inhibitor pargyline (open squares) and hordenine (closed triangles), while it was increased in the presence of methylamine (open circles). Each point is the mean ± SEM of 6 preparations from overweight subjects.
Taken together, these observations indicated that hordenine interacted with human MAO but did not modify SSAO activity. They also suggested that hordenine behaved as false substrate, or at least was not readily catabolized by MAO-A and SSAO, predominant in human fat depots.
The above in vitro experiments showed that hordenine exerts direct effect on adipose cells, but these effects are limited to an interplay with amine oxidation while they are not directly promoting or limiting triglyceride breakdown.
It was not astonishing to observe that hordenine interacts with MAO, since it shares chemical and structural similitudes with the physiological MAO substrate tyramine and with catecholamines that are catabolized by MAO both in brain and in peripheral tissues. In fact, hordenine has been described to be deaminated by rat MAO-B several decades ago [29], while its behavior towards human MAO-A or MAO-B remains poorly defined. If one considers that the inhibition of radiolabeled tyramine oxidation by human AT homogenates is demonstrative of a MAO-B inhibition, our study extrapolates to humans the pioneering characterization of Barwell and coworkers [29]. However, human adipose cells express more MAO-A than MAO-B, as confirmed here by the stronger inhibitory property of the MAO-A blocker Ro-11049 when compared to the MAO-B inhibitor. Due to such predominance of MAO-A in human AT, and to the similitude of inhibition curves between a substrate and an inhibitor (tyramine and Ro-166491, respectively), other interpretations rather than MAO inhibition could be raised from the inhibition of [14C]-tyramine oxidation by hordenine. Indeed, any single MAO substrate could have generated the same effect (as it was the case for cold tyramine). However, hordenine did not produce substantial amounts of hydrogen peroxide when incubated with human AT homogenates. Hordenine was far less efficient than tyramine, benzylamine and methylamine in generating this end-product of amine oxidase activity. Hordenine cannot be considered as a valuable substrate but rather as an inhibitor of amine oxidase activity. Under similar conditions, we observed that the naturally occurring methylxanthine caffeine interacted in an inhibitory manner with both the flavoenzyme MAO and the copper-containing SSAO. These observations thereby confirm the previously reported MAO-inhibitory [27] and SSAO-inhibitory [30] actions of caffeine. Although SSAO inhibition has been invoked in the anti-obesity properties of caffeine [31], we have recently reported that such mechanism is not implied in its antilipogenic effects [21]. Similarly, the present study tends to demonstrate that SSAO inhibition does not support the anti-obesity action of hordenine, if any.
Differently from caffeine, known to be a direct lipolytic agent on adipocytes [32], hordenine did not stimulate triglyceride breakdown, at least in mouse fat cells and under our experimental conditions. Such lack of in vitro effect is in apparent contradiction with the promised lipid mobilizing properties of hordenine-containing dietary supplements sold to obese subjects or fitness practitioners [3,8,33]. However, a limitation of our study is that it can reveal only rapid and acute lipolytic properties (similar to that of isoprenaline, ACTH or forskolin). In fact, in vivo mobilization can be obtained not solely by agents that directly interact with the management of triglyceride stores in adipocytes, but also by factors that indirectly stimulate lipolysis or fatty acid oxidation via neurohormonal loops, such as activation of sympathetic system. Our observations can only exclude hordenine as a direct strong activator of lipolysis, while other putative indirect effects, such as activation of the orthosympathetic tone, cannot be ruled out.
Nevertheless, the lack of reproduction by hordenine of the lipolytic effect of isoprenaline (a pan-agonist active at all the three subtypes of beta-adrenoceptors present on mouse adipocytes) does not agree with the description of the b2-AR agonist activity of hordenine reported on transfected cells [34]. This apparent discrepancy may be explained by the fact that receptors overexpressed in host cells can trigger much more signal transduction and amplification than in the native cells used here. In the mouse adipocytes, hordenine did not behave as a full agonist, irrespective of the level of b-adrenergic responsiveness of the target cells, exemplified here by the different efficiency of isoprenaline (high in young mice and slightly altered with aging). Since hordenine was also devoid of antilipolytic effect, alone or in combination with insulin, it looks unlikely that it can activate membrane receptors known to promote antilipolytic responses, such as purinergic receptors or a2-ARs [35]. However, on a pharmacological point of view, it cannot be excluded that the lack of effect of hordenine on adipocyte lipolysis is the result of a balance between an equivalent activation of lipolytic and antilipolytic components. In this view, hordenine has also been described as D2-dopaminergic biased agonist able to lower cAMP levels [16]. Unfortunately, dopamine is not a major immediate regulator of triglyceride assembly and breakdown in adipocytes [36]. Anyhow, hordenine did not exhibit any clear-cut trend to increase or to decrease basal triglyceride breakdown at doses ranging from 1 µM to 1 mM, while dualistic agents generally exhibit biphasic curves with inhibitory and activatory components (e.g. adrenaline, with stimulates both antilipolytic a2-AR and lipolytic b-AR [12]).
Closely related to hordenine, tyramine is not lipolytic save through the catecholamine release it induces in peripheral tissues, creating a somewhat stress-like adrenergic activation of triglyceride breakdown. In this view, tyramine is able, when administered in situ, to slightly activate lipolysis in human AT [37], while when administered in vitro directly on adipocytes it is not active [37] and rather antilipolytic at high doses [11]. Although not investigated in this study, a direct activation by hordenine of lipolysis in human adipocytes remains unlikely. Thus, we do not recommend toqualify hordenine as lipolytic agent. If this plant alkaloid really exerts beneficial effects on lipid handling and body weight control on consumers, these are probably not supported by direct stimulation of lipid mobilization. If demonstrated, the advertised action of hordenine on lipid burning, will likely result from its biotransformation into another more active substance, or alternatively from altered degradation of neurotransmitters by peripheral or central MAOs. To our knowledge, the gastrin releasing properties of hordenine and its capacity to bind serotonin receptor 4b [38] have not to play an essential role in the activation of lipolysis since many of the gastrointestinal peptides do not affect lipolysis in human adipocytes [39] and since serotonin is rather inhibitory of lipolysis [40].
The plasma levels of hordenine do not exceed 20 nM after a single ingestion of 1 L of beer in healthy consumers [16], and one can considered that the highest concentrations tested in the present in vitro experiments are extraphysiological. However, even at the lowest dose tested, hordenine did not modulate lipolysis. It cannot be conceived that oral intake of this biased D2 agonist/MAO inhibitor acutely regulates lipid mobilization from fat stores in human subjects. Clinical studies are required to demonstrate whether nutritional supplementation with hordenine is efficient to promote slimming effect in consumers. Nevertheless, in such studies it will be also of interest to have a comparative approach with other biogenic amines that often accompany hordenine in various edible plants (e.g. synephrine, octopamine, N-methyltyramine in Citrus fruits [2,5,41]). An addition or even a synergism between the actions of such dietary components is highly probable and may lead to beneficial long-term nutrigenomic effects occurring with repeated ingestion. However, when returning to the rapid and acute effects on adipocytes as target cells, and when compared with the intrinsic lipolytic effects of synephrine [6], hordenine is not expected to be a solid candidate to prevent excessive body weight gain.
Finally, it must be noted that MAO-interacting agents are not mandatorily slimming agents [42]. Although the MAO-B inhibitor selegiline has been recently reported to limit adiposity in rats [43], as it was also the case for phenelzine [44], the MAO substrate tyramine has quite opposite effects. Tyramine potentiates the poor antilipolytic effect of insulin in adipocytes from old rats [45] and potentiates the stimulation of glucose transport by vanadium in rodent fat cells [46]. Tyramine is also antilipolytic in human adipocytes [11] and its chronic administration in rodents transiently favors glucose homeostasis [47] at the expense of increased fattening [10]. In view of the risk of hypertensive crisis it represents, tyramine, a parent molecule of hordenine (which is N,N-dimethyltyramine) has never been claimed as a slimming or a 'fat-burning' agent, while this is the case for another related plant alkaloid, synephrine, known to be lipolytic [7] and devoid of serious adverse effects [5]. Similarly, to tyramine [47,48], hordenine has been reported to protect again diabetic complications in mice [49], but its antihyperglycaemic action is far for being demonstrated.
Differently from the reported actions of tyramine, hordenine did not exhibit antilipolytic actions in mouse adipocytes and did not generate hydrogen peroxide in AT homogenates. In the present in vitro study, hordenine also behaved differently from its parent molecule synephrine. Although closely related to other biogenic amines, hordenine does not appear to share the same metabolic influences. Hordenine has to be considered differently from tyramine, N-methyltyramine and synephrine because it is not so abundant than these other phytochemicals in several edible plants (e.g. Citrus fruits [7]) or nutritional supplements sold to control body weight, and most importantly because it does not appear to interfere so substantially with lipid handling or carbohydrate metabolism.
An interaction with human MAO is the sole effect of hordenine detected in this study. Together with its poor capacity to generate hydrogen peroxide when oxidized by human AT, this effect suggests that this phytochemical can be added to the long list of naturally occurring weak MAO-inhibitors. In fact, our findings tend to classify this amine as a mixed MAO substrate/inhibitor. This is in agreement with the overall qualification of indirect adrenergic drug already reported in previous pharmacological studies. Even if negative regarding direct activation of triglyceride breakdown, our in vitro observations indicate that hordenine could affect the sympathetic nervous system. Lastly, the hordenine consumption cannot likely increase lipid mobilization from fat depots since this plant alkaloid is lacking noticeable lipolytic action, even when tested at high doses.
This study was partly supported by recurrent grant from INSERM to Units 1048 & 1297. Authors thank all the staff of the Plastic surgery department (head: Jean-Louis Grolleau) of Rangueil University Hospital (Toulouse, France) for providing us with human adipose samples.
There is no conflict of interest to declare for any author of this work.
- Sommer T, Dlugash G, Hübner H, Gmeiner P, Pischetsrieder M (2019) Monitoring of the dopamine D2 receptor agonists hordenine and N-methyltyramine during the brewing process and in commercial beer samples. Food Chem 276: 745-753. [Crossref]
- Nelson BC, Putzbach K, Sharpless KE, Sander LC (2007) Mass spectrometric determination of the predominant adrenergic protoalkaloids in bitter orange (Citrus aurantium). J Agric Food Chem 55: 9769-9775. [Crossref]
- Viana C, Zemolin GM, Müller LS, Dal Molin TR, Seiffert H, et al. (2016) Liquid chromatographic determination of caffeine and adrenergic stimulants in food supplements sold in Brazilian e-commerce for weight loss and physical fitness. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 33: 1-9. [Crossref]
- Avula B, Bae JY, Chittiboyina AG, Wang Y, Wang M, et al. (2019) Liquid chromatography-quadrupole time of flight mass spectrometric method for targeted analysis of 111 nitrogen-based compounds in weight loss and ergogenic supplements. J Pharm Biomed Anal 174: 305-323. [Crossref]
- Stohs SJ, Preuss HG, Shara M (2011) The safety of Citrus aurantium (bitter orange) and its primary protoalkaloid p-synephrine. Phytother Res 25: 1421-1428. [Crossref]
- Mercader J, Wanecq E, Chen J, Carpéné C (2011) Isopropylnorsynephrine is a stronger lipolytic agent in human adipocytes than synephrine and other amines present in Citrus aurantium. J Physiol Biochem 67: 443-452. [Crossref]
- Carpéné MA, Testar X, Carpéné C (2014) High doses of synephrine and octopamine activate lipolysis in human adipocytes, indicating that amines from Citrus might influence adiposity. In: Citrus K Hayat, Editor Nova Science Publishers Inc Chapter 8: 141-168.
- Biesterbos JWH, Sijm D, van Dam R, Mol HGJ (2019) A health risk for consumers: the presence of adulterated food supplements in the Netherlands. Food Addit Contam Part A Chem Anal Control Expo Risk Assess 36: 1273-1288. [Crossref]
- Visentin V, Prevot D, Marti L, Carpéné C (2003) Inhibition of rat fat cell lipolysis by monoamine oxidase and semicarbazide-sensitive amine oxidase substrates. Eur J Pharmacol 466: 235-243. [Crossref]
- Les F, Iffiu-Soltesz Z, Mercader J, Carpéné C (2017) Tyramine activates lipid accumulation in rat adipocytes: influences of in vitro and in vivo administration. AIMS Molecular Science 4: 339-351.
- Carpéné C, Galitzky J, Belles C, Zakaroff-Girard A (2018) Mechanisms of the antilipolytic response of human adipocytes to tyramine, a trace amine present in food. J Physiol Biochem 74: 623-633. [Crossref]
- Lafontan M, Berlan M (1995) Fat cell alpha 2-adrenoceptors: the regulation of fat cell function and lipolysis. Endocr Rev 16: 716-738. [Crossref]
- Tipton KF (2018) 90 years of monoamine oxidase: some progress and some confusion. J Neural Transm (Vienna) 125: 1519-1551. [Crossref]
- Morin N, Lizcano JM, Fontana E, Marti L, Smih F, et al. (2001) Semicarbazide-sensitive amine oxidase substrates stimulate glucose transport and inhibit lipolysis in human adipocytes. J Pharmacol Exp Ther 297: 563-572. [Crossref]
- Carpéné C, Mauriège P, Boulet N, Biron S, Grolleau J, et al. (2019) Methylamine Activates Glucose Uptake in Human Adipocytes Without Overpassing Action of Insulin or Stimulating its Secretion in Pancreatic Islets. Medicines (Basel) 6: 89. [Crossref]
- Sommer T, Göen T, Budnik N, Pischetsrieder M (2020) Absorption, Biokinetics, and Metabolism of the Dopamine D2 Receptor Agonist Hordenine (N,N-Dimethyltyramine) after Beer Consumption in Humans. J Agric Food Chem 68: 1998-2006. [Crossref]
- Kim SC, Lee JH, Kim MH, Lee J, Beom Kim Y, et al. (2013) Hordenine, a single compound produced during barley germination, inhibits melanogenesis in human melanocytes. Food Chem 141: 174-181.
- Iffiu-Soltesz Z, Wanecq E, Lomba A, Portillo MP, Pellati F, et al. (2010) Chronic benzylamine administration in the drinking water improves glucose tolerance, reduces body weight gain and circulating cholesterol in high-fat diet-fed mice. Pharmacol Res 61: 355-363. [Crossref]
- Carpéné C, Galitzky J, Saulnier-Blache JS (2016) Short-term and rapid effects of lysophosphatidic acid on human adipose cell lipolytic and glucose uptake activities. AIMS Molecular Science 3: 222-237.
- Leroux M, Lemery T, Boulet N, Briot A, Zakaroff A, et al. (2019) Effects of the amino acid derivatives, β-hydroxy-β-methylbutyrate, taurine, and N-methyltyramine, on triacylglycerol breakdown in fat cells. J Physiol Biochem 75: 263-273. [Crossref]
- Haj Ahmed W, Boulet N, Briot A, Ryan BJ, Kinsella GK, et al. (2021) Novel Facet of an Old Dietary Molecule? Direct Influence of Caffeine on Glucose and Biogenic Amine Handling by Human Adipocytes. Molecules 26: 3831. [Crossref]
- Fowler CJ, Tipton KF (1981) Concentration dependence of the oxidation of tyramine by the two forms of rat liver mitochondrial monoamine oxidase. Biochem Pharmacol 30: 3329-3332. [Crossref]
- Carpéné C, Les F, Hasnaoui M, Biron S, Marceau P, et al. (2016) Anatomical distribution of primary amine oxidase activity in four adipose depots and plasma of severely obese women with or without a dysmetabolic profile. J Physiol Biochem 73: 475-486. [Crossref]
- Prévot D, Soltesz Z, Abello V, Wanecq E, Valet P, et al. (2007) Prolonged treatment with aminoguanidine strongly inhibits adipocyte semicarbazide-sensitive amine oxidase and slightly reduces fat deposition in obese Zucker rats. Pharmacol Res 56: 70-79. [Crossref]
- Haj Ahmed W, Peiro C, Fontaine J, Ryan BJ, Kinsella GK, et al. (2020) Methylxanthines Inhibit Primary Amine Oxidase and Monoamine Oxidase Activities of Human Adipose Tissue. Medicines (Basel) 7: 18. [Crossref]
- Pizzinat N, Marti L, Remaury A, Leger F, Langin D, et al. (1999) High expression of monoamine oxidases in human white adipose tissue: evidence for their involvement in noradrenaline clearance. Biochem Pharmacol 58: 1735-1742. [Crossref]
- Petzer A, Pienaar A, Petzer JP (2013) The interactions of caffeine with monoamine oxidase. Life Sci 93: 283-287. [Crossref]
- Olivieri A, Rico D, Khiari Z, Henehan G, O'Sullivan J, et al. (2011) From caffeine to fish waste: amine compounds present in food and drugs and their interactions with primary amine oxidase. J Neural Transm (Vienna) 118: 1079-1089. [Crossref]
- Barwell CJ, Basma AN, Lafi MA, Leake LD (1989) Deamination of hordenine by monoamine oxidase and its action on vasa deferentia of the rat. J Pharm Pharmacol 41: 421-423. [Crossref]
- Olivieri A, Tipton K (2011) Inhibition of bovine plasma semicarbazide-sensitive amine oxidase by caffeine. J Biochem Molec Toxicol 25: 26-27. [Crossref]
- Papukashvili D, Rcheulishvili N, Deng Y (2020) Attenuation of Weight Gain and Prevention of Associated Pathologies by Inhibiting SSAO. Nutrients 12: 184. [Crossref]
- Gomez-Zorita S, Belles C, Briot A, Fernández-Quintela A, Portillo MP, et al. (2017) Pterostilbene Inhibits Lipogenic Activity similar to Resveratrol or Caffeine but Differently Modulates Lipolysis in Adipocytes. Phytother Res 31: 1273-1282. [Crossref]
- Inchiosa MA (2011) Experience (Mostly Negative) with the Use of Sympathomimetic Agents for Weight Loss. J Obesity 2011: 764584. [Crossref]
- Chikazawa M, Sato R (2018) Identification of Functional Food Factors as β2-Adrenergic Receptor Agonists and Their Potential Roles in Skeletal Muscle. J Nutr Sci Vitaminol (Tokyo) 64: 68-74. [Crossref]
- Castan I, Valet P, Quideau N, Voisin T, Ambid L, et al. (1994) Antilipolytic effects of alpha 2-adrenergic agonists, neuropeptide Y, adenosine, and PGE1 in mammal adipocytes. Am J Physiol 266: R1141-1147. [Crossref]
- Carpéné C, Galitzky J, Fontana E, Atgié C, Lafontan M, et al. (1999) Selective activation of beta3-adrenoceptors by octopamine: comparative studies in mammalian fat cells. Naunyn Schmiedebergs Arch Pharmacol 359: 310-321. [Crossref]
- Adams F, Boschmann M, Schaller K, Franke G, Gorzelniak K, et al. (2006) Tyramine in the assessment of regional adrenergic function. Biochem Pharmacol 72: 1724-1729. [Crossref]
- Yasi EA, Allen AA, Sugianto W, Peralta-Yahya P (2019) Identification of Three Antimicrobials Activating Serotonin Receptor 4 in Colon Cells. ACS Synth Biol 8: 2710-2717. [Crossref]
- Richter WO, Schwandt P (1985) Glycerol release from incubated human adipocytes is not affected by gastrointestinal peptides. Int J Obes 9: 25-27. [Crossref]
- Hansson B, Medina A, Fryklund C, Fex M, Stenkula KG (2016) Serotonin (5-HT) and 5-HT2A receptor agonists suppress lipolysis in primary rat adipose cells. Biochem Biophys Res Commun 474: 357-363. [Crossref]
- Pellati F, Benvenuti S (2007) Chromatographic and electrophoretic methods for the analysis of phenethylamine [corrected] alkaloids in Citrus aurantium. J Chromatogr A 1161: 71-88. [Crossref]
- Song MS, Matveychuk D, MacKenzie EM, Duchcherer M, Mousseau DD, et al. (2013) An update on amine oxidase inhibitors: multifaceted drugs. Prog Neuropsychopharmacol Biol Psychiatry 44: 118-124. [Crossref]
- Nagy CT, Koncsos G, Varga ZV, Baranyai T, Tuza S, et al. (2018) Selegiline reduces adiposity induced by high-fat, high-sucrose diet in male rats. Br J Pharmacol 175: 3713-3726. [Crossref]
- Carpéné C, Mercader J, Le Gonidec S, Schaak S, Mialet-Perez J, et al. (2018) Body fat reduction without cardiovascular changes in mice after oral treatment by the MAO inhibitor phenelzine. Br J Pharmacol 175: 2428-2440. [Crossref]
- Bairras C, Ferrand C, Atgié C (2003) Effect of tyramine, a dietary amine, on glycerol and lactate release by isolated adipocytes from old rats. J Physiol Biochem 59: 161-167.
- Marti L, Morin N, Enrique-Tarancon G, Prevot D, Lafontan M, et al. (1998) Tyramine and vanadate synergistically stimulate glucose transport in rat adipocytes by amine oxidase-dependent generation of hydrogen peroxide. J Pharmacol Exp Ther 285: 342-349. [Crossref]
- Visentin V, Bour S, Boucher J, Prévot D, Valet P, et al. (2005) Glucose handling in streptozotocin-induced diabetic rats is improved by tyramine but not by the amine oxidase inhibitor semicarbazide. Eur J Pharmacol 522: 139-146. [Crossref]
- Lino CdS, Sales TdP, Gomes PB, do Amaral JF, Alexandre FSO, et al. (2007) Anti-diabetic activity of a fraction from Cissus verticillata and tyramine, its main bioactive constituent, in alloxan-induced diabetic rats. Am J Pharmacol Toxicol 2: 178-188.
- Su S, Cao M, Wu G, Long Z, Cheng X, et al. (2018) Hordenine protects against hyperglycemia-associated renal complications in streptozotocin-induced diabetic mice. Biomed Pharmacother 104: 315-324. [Crossref]




