Abstract
Objectives: This study designed to evaluate the efficacy and tolerability of heme iron polypeptide (HIP) in treatment of iron deficiency anemia during pregnancy.
Methods: 150 pregnant women with hemoglobin <10 gm/dl due to iron deficiency included in this study and treated with HIP for correction of iron deficiency anemia during pregnancy. Treatment efficacy checked by comparing the pre-treatment values of hemoglobin, serum ferritin, reticulocytes, mean corpuscular volume (MCV) and mean corpuscular hemoglobin (MCH) by the 3-months` post-treatment values.
Results: The mean pre-treatment hemoglobin significantly increased from 8.8 ± 3.7 to 11.4 ± 3.04 gm/dl and the mean pretreatment ferritin level significantly increased from 15.6 ± 6.3 to 118.4 ± 4.6 ug/l 3-months` after Proferrin treatment. In addition; the mean pre-treatment RBCs MCV significantly increased from 72.6 ± 5.2 to 92.1 ± 3.8 FL, and the mean -treatment RBCs MCH significantly increased from 24.5 ± 8.1 to 25.8 ± 6.6 pg 3-months` after Proferrin treatment. While, the mean pre-treatment reticulocytes count significantly decreased from 3.4 ± 4.2 to 1.2 ± 3.3 106/mm3 3-months` after Proferrin treatment.
Conclusion: HIP is safe, tolerable, effective, oral iron preparation to treat iron deficiency anemia with pregnancy; it increases the hemoglobin and replaces the depleted iron store.
Introduction
The World Health Organization defined hemoglobin below 11 gm/dl as anemia. Anemia is a public health problem and a direct cause of disability. Fifty-two percent (52%) of pregnant women in developing countries suffering from anemia compared to 23% in developed countries [1].
Causes of anemia include; iron deficiencies, poor nutrition, malabsorption, hookworm infestation, schistosomiasis, human immune deficiency (HIV) and hemoglobinopathies [1,2]. There is a high demand for iron during pregnancy (average 600 mg) and on top of the demands of pregnancy is the inevitable blood loss during deliveries [3,4].
A blood loss of ≥1 Liter occurs in 7% of vaginal deliveries and 23% of cesarean deliveries associated with 1000-1500 ml blood loss [3,4]. Maternal anemia is a leading cause of perinatal morbidity, adverse outcome in obstetrics, maternal mortality and blood transfusion [5-8]. Nissenson et al. [9] found that 6 months after evaluation of HIP (Proferrin®-ES) in hemodialysis patients who had been on maintenance intravenous iron therapy, the intravenous iron was discontinued and replaced with oral HIP. This study designed to evaluate the efficacy and tolerability of HIP (Proferrin®-ES) in treatment of iron deficiency anemia during pregnancy.
Methods
This comparative multicenter study conducted over 6 months in three private hospitals in Kuwait (Royal Hayat, Al Seef and Hadi), after approval of the study by the hospitals ethical committee. One hundred and fifty (150) pregnant women with hemoglobin level below 10 gm/dl due to iron deficiency anemia included in this study and treated with HIP (Proferrin®-ES) for correction of iron deficiency anemia during pregnancy after informed consent.
Inclusion criteria includes; pregnant women >18 years, 24-30 weeks` gestation with hemoglobin level between 8-10 gm/dl. Pregnant women with anemia due to causes other than iron deficiency and pregnant women received blood transfusion during current pregnancy excluded from this study. Eight (8) pregnant women excluded from this study because of travelling, preterm delivery and intolerance to Proferrin®-ES, so the study completed and statistical analysis done for one hundred and forty-two women (142).
Diagnosis of iron deficiency anemia confirmed by; hemoglobin concentration (gm/dl), serum ferritin (ug/l), mean Corpuscular Volume (MCV) and mean corpuscular hemoglobin (MCH) [6-8]. Heme Iron Polypeptide (Proferrin®-ES), (Nexgen Pharma Inc, Coloeado, USA) derived from bovine hemoglobin and it has unique carrier intestinal receptors Heme Carrier Protein-1 (HCP-1). HIP peptides content of the Proferrin tablets enhance the solubility of HIP and increase the bioavailable iron for absorption.
According to the manufacturer instructions, the HIP (Proferrin®-ES) tablets given to the studied women twice daily (1 tablet morning and 1 tablet evening) not related to meals till hemoglobin level of 11-12 gms/dl, then one tablet daily as maintenance dose [9]. After oral intake, Proferrin®-ES tablets, the iron content of the tablets absorbed by the HCP-1 receptors of the small intestine and the serum peak of iron reached within 2-4 hours. Each tablet of Proferrin®-ES increases the serum iron by 3.15 mg [9]. Oral folic acid given to the studied women with Proferrin®-ES to avoid folic deficiency and participants asked during each ante-natal care visit for any side effects related to Proferrin®-ES as gastrointestinal upset, metallic taste, constipation and/or intolerance. Efficacy of Proferrin®-ES treatment checked by comparing the pre-treatment by the 3-months` post-treatment of hemoglobin, ferritin, reticulocytes, MCV and MCH [10,11].
Sample size and statistical analysis
G* Power software used for calculation of the studied sample size, statistical analysis done using statistical package for social sciences (SPSS) version 20 (Chicago, IL, USA) and the Student’s t-test used for quantitative data analysis. The significance level set as p<0.05.
Results
One hundred and fifty (150) pregnant women with hemoglobin level below 10 gm/dl due to iron deficiency anemia were included in this study and treated with HIP (Proferrin®-ES) for correction of iron deficiency anemia during pregnancy. Eight (8) pregnant women excluded from this study because of travelling (3 women), preterm delivery (2 women) and intolerance to Proferrin®-ES (3 women), so the study completed with one hundred and forty-two pregnant women (142) (Figure 1).
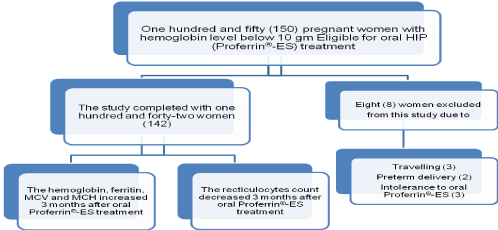
Figure 1. The study design and the results 3 months` after oral HIP (Proferrin®-ES) treatment.
The mean age of the studied women was 25.4 ± 2.3, mean parity was 4.6 ± 6.3, mean weight was 82.6 ± 4.5, and the mean gestational age was 26.4 ± 3.3 weeks` gestation. The mean pre-treatment hemoglobin significantly increased from 8.8 ± 3.7 to 11.4 ± 3.04 gm/dl and the mean pre-treatment ferritin level significantly increased from 15.6 ± 6.3 to 118.4 ± 4.6 ug/l (p<0.01 and <0.001; respectively) 3-months` after Proferrin®-ES treatment.
In addition; the mean pre-treatment RBCs MCV significantly increased from 72.6 ± 5.2 to 92.1 ± 3.8 FL and the mean pre-treatment RBCs MCH significantly increased from 24.5 ± 8.1 to 25.8 ± 6.6 pg (p<0.001 and <0.01; respectively) 3-months` after Proferrin®-ES treatment. While, the mean pre-treatment reticulocytes count significantly decreased from 3.4 ± 4.2 to 1.2 ± 3.3 106/mm3 (p<0.01) 3-months` after Proferrin®-ES treatment (Table 1).
Table 1. Pre-treatment hemoglobin, reticulocytes count, ferritin level, MCV and MCH compared to 3-months` post-treatment values.
Variables |
Pre-treatment values |
3-months` Post-treatment values |
P value (95% CI) Significance |
Hemoglobin (gm/dl) |
8.8 ± 3.7 |
11.4 ± 3.04 |
0.01* (-3.4, -2.6, -1.8) |
Reticulocytes count (106/mm3) |
3.4 ± 4.2 |
1.2 ± 3.3 |
0.002* (1.3, 2.2, 3.07) |
Ferritin level (ug/l) |
15.6 ± 6.3 |
118.4 ± 4.6 |
0.0001* (-104, -102.8, -101.5) |
RBCs MCV (FL) |
72.6 ± 5.2 |
92.1 ± 3.8 |
0.0001* (-20.5, -19.5, -18.4) |
RBCs MCH (pg) |
24.5 ± 8.1 |
25.8 ± 6.6 |
0.007* (-3.0, -1.3, 0.4) |
*= significant difference; CI = Confidence interval; Data presented as mean and ± SD; MCH = Mean corpuscular hemoglobin; MCV = Mean corpuscular volume; RBCS = Red blood cells; Student’s t-test used for statistical analysis.
Only 2.1% (3/142) of the studied women developed gastrointestinal intolerance and upset with oral Proferrin®-ES (insignificant difference and excluded from the study) and no other side effects recorded with oral Proferrin®-ES.
Discussion
The inevitable blood loss during deliveries aggravates maternal anemia and increases the need for blood transfusion [3,4,7,8]. One hundred and fifty pregnant women with hemoglobin level below 10 gm/dl due to iron deficiency anemia were included in this multicenter study and treated with HIP (Proferrin®-ES) for correction of iron deficiency anemia during pregnancy. Eight (8) pregnant women excluded from this study; because of travelling, preterm delivery and intolerance to Proferrin®-ES and the study completed with one hundred and forty-two pregnant women (142).
The mean pre-treatment hemoglobin and ferritin levels significantly increased 3-months` after Proferrin®-ES treatment. In addition; the mean pre-treatment RBCs MCV and MCH significantly increased 3-months` after Proferrin®-ES treatment. While, the mean pre-treatment reticulocytes count significantly decreased 3-months` after treatment.
Barraclough et al. [12] designed a multicenter study in 2009; to provide evidence to the nephrologists and their peritoneal dialysis whether HIP (Proferrin®-ES) administration effectively augments the iron stores than conventional oral iron supplementation or not [12].
2021 Copyright OAT. All rights reserv
Barraclough et al. [13], concluded that; HIP showed no clear safety or efficacy in peritoneal dialysis patients compared with conventional oral iron supplements. The reduction in serum ferritin levels and high costs associated with HIP (Proferrin®-ES) therapy suggest that this agent is unlikely to have a significant role in iron supplementation in peritoneal dialysis patients.
Although, Barraclough et al. [13] concluded that HIP (Proferrin®-ES) showed no clear safety or efficacy in peritoneal dialysis patients compared with conventional oral iron supplements. Nissenson et al. [9], found that 6 months after evaluation of HIP (Proferrin®-ES) in hemodialysis patients who had been on maintenance intravenous iron therapy, the intravenous iron was discontinued and replaced with oral HIP.
In addition; this study concluded that the hemoglobin, ferritin, MCV and MCH significantly increased 3-months` after Proferrin®-ES treatment in anemic pregnant women. Moreover; significant decrease in reticulocytes count observed 3-months` after Proferrin®-ES treatment. Gastrointestinal side effects are very common problem with oral iron preparations. Al Momen et al. [14], in their study compared 52 women treated with intravenous iron sucrose and 59 women treated with 300 mg oral iron sulfate, found that 18 (30 %) of the oral iron group complained of disturbing gastrointestinal symptoms and 18 (30 %) had poor compliance [14].
While, in this study; only 2.1% (3/142) of the studied women developed gastrointestinal intolerance and upset with oral Proferrin®-ES (insignificant difference and excluded from the study) and no other side effects recorded with oral Proferrin®-ES. The anemic pregnant women developed gastrointestinal disturbance with Proferrin®-ES treated with intravenous iron sucrose because they cannot tolerate oral iron preparation other than Proferrin®-ES.
To the best our Knowledge, the current study was the first study designed and conducted to evaluate the efficacy and tolerability of heme iron polypeptide (HIP) in treatment of iron deficiency anemia during pregnancy. The limited data and studies available about HIP (Proferrin®-ES) was the only limitation faced during conduction of this study. More comparative studies needed to compare the efficacy of HIP (Proferrin®-ES) in treatment of iron deficiency anemia during pregnancy with the available oral or intravenous iron preparations.
Conclusion
HIP is safe, tolerable, effective, oral iron preparation to treat iron deficiency anemia with pregnancy; it increases the hemoglobin and replaces the depleted iron store.
Acknowledgments
Authors grateful to women agreed to participate in this study.
Declaration of interest
Authors declare no conflict of interest related to this study.
Financial disclosure
Authors themselves funded study.
References
- Güleç UK, Özgünen FT, Evrüke IC, Demir SC (2013) Anemia in Pregnancy. Arch Med Rev J 22: 300-316.
- Tunç SY, Goruk NY, Ceylan B, Tunç N (2012) The relationship between gestation and iron deficiency anemia in women applied to gynecologic outpatient clinic. J Clin Exp Invest 3: 49-52.
- Stafford I, Dildy GA, Clark SL, Belfort MA (2008) Visually estimated and calculated blood loss in vaginal and cesarean delivery. Am J Obstet Gynecol 199: 519. [Crossref]
- Barut A and Harma M (2009) Intravenous iron treatment for iron deficiency anemia in pregnancy. J Turkish-German Gynecol Assoc 10: 109-15.
- Kalaivani K (2009) Prevalence & consequences of anaemia in pregnancy. Indian J Med Res 130: 627-633. [Crossref]
- Shafi D, Purandare SV, Sathe AV (2012) Iron Deficiency Anemia in Pregnancy: Intravenous Versus Oral Route. J Obstet Gynaecol India 62: 317-321. [Crossref]
- Abdelazim IA, Abu-Faza M, Sanchez VJ (2014) Iron Therapy in Treatment of Iron Deficiency Anemia Before Delivery: Intravenous Versus Oral Route. J Gynecol Obstet Med 1: 1-7.
- Abdelazim IA, Abu-Faza ML, Hamdan SB(2016) Intravenous Iron Saccharate Infusion for Treatment of Iron Deficiency. ARC J Gynecol Obstet 1: 16-20.
- Nissenson AR, Berns JS, Sakiewicz P, Ghaddar S, Moore GM, et al. (2003) Clinical evaluation of heme iron polypeptide: sustaining a response to rHuEPO in hemodialysis patients. Am J Kidney Dis 42: 325-330. [Crossref]
- Yee J, Besarab A (2002) Iron sucrose: the oldest iron therapy becomes new. Am J Kidney Dis 40: 1111-1121. [Crossref]
- Teucher B, Olivares M, Cori H (2004) Enhancers of iron absorption: ascorbic acid and other organic acids. Int J Vitam Nutr Res 74: 403-419. [Crossref]
- Barraclough KA, Noble E, Leary D, Brown F, Hawley CM, et al (2009) Rationale and design of the oral HEMe iron polypeptide Against Treatment with Oral Controlled Release Iron Tablets trial for the correction of anaemia in peritoneal dialysis patients (HEMATOCRIT trial). BMC Nephrol 10: 20. [Crossref]
- Barraclough KA, Brown F, Hawley CM, Leary D, Noble E, et al (2012) A randomized controlled trial of oral heme iron polypeptide versus oral iron supplementation for the treatment of anaemia in peritoneal dialysis patients: HEMATOCRIT trial. Nephrol Dial Transplant 27: 4146-4153. [Crossref]
- al-Momen AK, al-Meshari A, al-Nuaim L, Saddique A, Abotalib Z, et al (1996) Intravenous iron sucrose complex in the treatment of iron deficiency anemia during pregnancy. Eur J Obstet Gynecol Reprod Biol 69: 121-124. [Crossref]

