Abstract
To evaluate the roles of the glycine transporter-1 (GLYT1) in proliferation of neural stem/progenitor cells (NPCs) in the embryonic hippocampus, we examined the effects of a GLYT1 inhibitor on proliferative activity in the NPCs. NPCs were prepared from the hippocampus of 15-days-old embryonic mice by culturing in DMEM/F12 medium with EGF and bFGF. Double-immunostaining revealed that the cells expressed GLYT1 and nestin. The cells were cultured for 6 days in vitro (DIV) in the absence or presence of N[3-(4-fluorophenyl)-3-(4-phenylphenoxy)propyl] sarcosine (NFPS, a non-transportable inhibitor of GLYT1). Treatment with NFPS led to a significant decrease in the number of surviving cells cultured for 6 DIV, in a concentration-dependent manner, from above 1 μM. However, NFPS had no significant cell toxicity, at least during a 1-day treatment. In addition, ELISA of 5'-bromo-2'-deoxyuridine (BrdU) revealed that treatment with NFPS resulted in a marked decrease in proliferative activity. These results suggest that GLYT1 could positively regulate proliferative activity of NPCs derived from the hippocampi of embryonic mice.
Keywords
Glycine transporter; Neural stem/progenitor cell; Neurogenesis; Proliferation
Introduction
Neural stem/progenitor cells (NPCs), defined by their capacity for self-renewal and differentiation into 3 major cell types, i.e., neurons, astrocytes, and oligodendrocytes, play an essential role in the development and maturation of the central nervous system.
NPCs can be isolated from rodent fetal tissues and maintained in culture as spherical aggregates of undifferentiated cells termed “neurospheres” [1,2]. To elucidate the full potential of NPCs, it is essential to understand physiological pathways and extrinsic factors that control their proliferation and differentiation.
NPCs are present not only in the developing brain, but also in the adult brain in different areas with neurogenic potential [3]. Although the importance of NPCs in the adult brain is uncertain, accumulating evidence has suggested that the capability for self-renewal would be important for normal brain functions, including, learning, memory, and emotional responses [4,5].
Glycine is a major inhibitory neurotransmitter in the spinal cord and brain stem and acts on strychnine-sensitive glycine receptor chloride channels to induce neuronal inhibition. The postsynaptic actions of glycine are terminated by the rapid reuptake mechanism, which is mainly mediated by glycine transporter-1 (GLYT1) and -2 (GLYT2). GLYT1 is disturbed more widely in the central nervous system, without restriction to glycinergic terminals, and has even been found in brain regions devoid of strychnine-sensitive receptors. Conversely, GLYT2 is selectively expressed in the spinal cord and brainstem. The glycine transporter is a potential pharmacological target for neurological disorders [6,7]. In addition, the glycine transporters may also be targets for pain treatment, since selective GLYT1/GLYT2 inhibitors produce analgesia in pain models [8]. Additionally, GLYT1 inhibitors may improve the cognitive deficits of patients with schizophrenia by increasing glycine levels around the N-methyl-D-aspartate (NMDA) receptors [6]. Interestingly, glycine is a co-agonist of NMDA receptors [9]; thus, both glycine receptors and NMDA receptors can be subject to modulation by GLYT1 [10-12]. However, the role of GLYT1 in the proliferation of NPCs remains largely unknown.
The present study evaluated the effect of a non-transportable inhibitor of GLYT1 N[3-(4-fluorophenyl)-3-(4-phenylphenoxy)propyl] sarcosine (NFPS) on proliferative activity in NPCs derived from the hippocampi of embryonic mice.
Materials and methods
Cell cultures
The protocol used in this study met the guidelines of The Japanese Society for Pharmacology and was approved by the Committee for Ethical Use of Experimental Animals at Setsunan University. Hippocampal NPC cultures were prepared from the hippocampi of 15-day-old embryonic mice, as originally described by Yoneyama et al. [2]. In brief, hippocampi were dissected from embryonic Std-ddY male mice and were then suspended in DMEM/F12 supplemented with 10% (v/v) fetal bovine serum (FBS). Cells were centrifuged at 500 × g for 5 min, and subsequently washed once again with DMEM/F12 containing 0.6% (w/v) glucose, 15 mM sodium bicarbonate, 20 nM progesterone, 30 nM sodium selenite, 60 nM putrescine, and 100 μg/mL apo-transferrin. Finally, the cells were suspended in growth medium consisting of DMEM/F12 containing 0.6% (w/v) glucose, 15 mM sodium bicarbonate, 20 nM progesterone, 30 nM sodium selenite, 60 nM putrescine, 100 μg/mL apo transferrin, 25 μg/mL insulin, 10 ng/mL epidermal growth factor and 10 ng/mL basic fibroblast growth factor. These cells were seeded at a density of 6 × 104 cells/mL on 6-well dishes (Greiner Bio-one, Frickenhausen, Germany) after counting viable cell numbers determined using the trypan blue exclusion test and they were cultured for a period up to 9 days in vitro (DIV) in the growth medium with a half medium change every 3 days as primary cultures of NPCs. The cells in the 9 DIV cultures were dispersed by using NeuroCult Chemical Dissociation Kit (StemCell Technologies Inc., UK), and then replated at a density of 6 × 104 cells/mL on 6-well or 24-well dishes as secondary cultures. The cells were kept in the growth medium for various time periods, up to 6 DIV under the same conditions as described for the primary cultures. Experiments in the present study were usually performed by using the secondary cultures unless otherwise indicated. The cultures were always maintained at 37°C in 95% (v/v) air/5% (v/v) CO2; and after seeding, the cells were exposed to no FBS at all to avoid possible influences of hitherto unidentified factors present in FBS.
Immunocytochemical analysis
Cells were fixed in 4% paraformaldehyde for 15 min at 4°C and then blocked with 5% (w/v) normal goat serum in Tris-buffered saline containing 0.03% Tween (0.03% TBST). Subsequently, the cells were incubated with appropriately diluted primary antibodies against nestin (Chemicon International, Temecula, CA) and GLYT1 (Alpha Diagnostics International, San Antonio, TX) overnight at 4°C. Finally, the cells were incubated with the corresponding secondary antibody, i.e., an anti-mouse IgG antibody conjugated with FITC or anti-rabbit IgG conjugated with Texas Red. After rinsing for 5 min with 0.03% TBST, the cells were observed under a DS-Ril camera (Nikon, Tokyo, Japan) attached to BX41 microscope (Olympus, Osaka, Japan). The number of immunoreactive cells in 4 different microscopic visual fields at a magnification of 20-fold was counted in each well on a plate.
Immunoblot analysis
Cells were harvested with ice-cold homogenizing buffer consisting of 10 mM Tris-HCl buffer (pH 7.5) containing 0.32 M sucrose, 1 mM EDTA, 1 mM EGTA, 5 mM dithiothreitol, phosphatase inhibitors (10 mM sodium b-glycerophosphate and 1 mM sodium orthovanadate), and 1 mg/mL each protease inhibitors [(p-amidinophenyl) methanesulfonyl fluoride, benzamidine, leupeptin, and antipain], followed by centrifugation at 4°C for 5 min at 15,000 × g. Pellets thus obtained were suspended and then homogenized in the same buffer using a sonicator. The cell lysates were boiled at 100°C for 10 min in 10 mM Tris-HCl buffer (pH 6.8) containing 10% (v/v) glycerol, 2% (w/v) SDS, 0.01% (w/v) bromophenol blue, and 5% (v/v) 2-mercaptoethanol, and were then stored at -80°C until required for use. An aliquot (10 mg protein) of the cell lysates was loaded onto a 5% (w/v) polyacrylamide gel, electrophoresed, and transferred to a polyvinylidene fluoride membrane.
Protein concentrations were measured using the Protein Assay Rapid kit (Wako Pure Chemical Industries, Ltd., Osaka, Japan). Immunoblot assays were performed using primary antibodies against GLYT1, as described previously [13].
MTT assay
The 3-[4,5-Dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) assay was used to determine cell viability. In brief, MTT solution (0.5 mg/mL in phosphate-buffered saline) was added to each well of the culture dishes, and then the cells were incubated for 2 h at 37°C. Subsequently, solubilizing solution (0.4 M HCl in isopropanol), equivalent to the MTT solution in volume, was added, after which the absorbance at 570 nm was measured.
5-Bromo-2′-deoxyuridine (BrdU) incorporation
Cell proliferation was assessed by evaluating BrdU incorporation into cells during the culture period. Cells were exposed to 0.1 µM BrdU for 12 h and then centrifuged at 300 × g for 10 min. After removing the medium, the BrdU levels in the cells remaining in the dish were determined using a Cell Proliferation ELISA kit according to the manufacturer’s instructions (Roche Diagnostics, Mannheim, Germany).
Propidium iodide uptake assay
The extent and distribution of dying cells were determined after replated cells for 1 DIV by adding propidium iodide (PI) at a final concentration of 2 mg/mL into the growth medium. Cells were incubated with PI for 10 min at 37ºC. Immediately after the incubation, the cells were observed under a VB-7010 digital camera (KEYENCE, Osaka, Japan) attached to a fluorescence microscope IX71 (Olympus). The number of PI-positive cells in 4 different microscopic visual fields at a magnification of 20 × was counted in each well on a plate.
Data analysis
All data were expressed as the mean ± S.E., and the statistical significance was determined using a 2-tailed Student’s t-test or one-way analysis of variance with the Bonferroni/Dunnett post hoc test.
Results
GLYT1 in the NPC
To confirm the expression of GLYT1 protein, immunostaining and immunoblot analyses were performed on NPCs derived from the hippocampi of embryonic mice. Immunoreactivity to GLYT1 antibody was seen in more than 95% of NPCs that were labeled with nestin (Figure 1). Immunoblot analysis revealed that the protein level of GLYT1 was the highest in cells at 0 DIV and progressively decreased during culturing to 6 DIV.
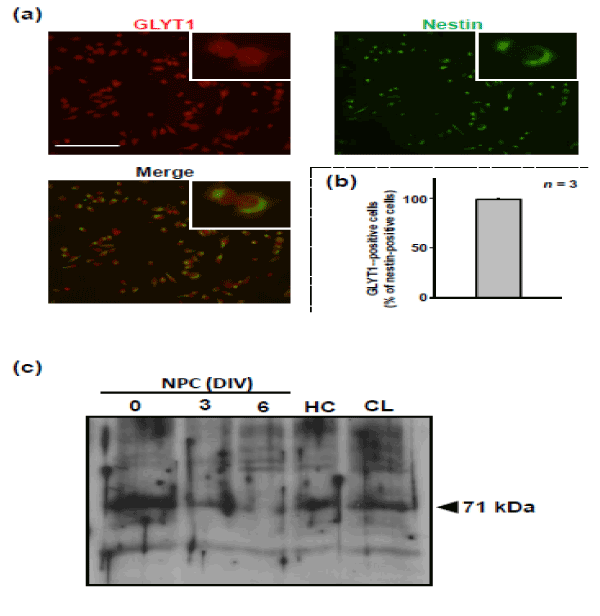
Figure 1: Expression of GLYT1 in NPC cultures. Cells were isolated from embryonic mouse hippocampus and then cultured in the growth medium for 9 DIV. At 9 DIV, the cells were harvested and dispersed for replating on dishes that had been previously coated with poly-L-lysine. (a) After having been incubated in the growth medium for 1 h, the cells were fixed for double-immunostaining for GLYT1 (red) and nestin (green). (b) The graph shows proportion of GLYT1 (+) cells in nestin (+) cells. Values are expressed mean ± S.E. from 3 independent experiments. (c) Cells were harvested for subsequent replating and culturing in the growth medium for 6 DIV. As positive controls, tissue lysates of hippocampus (HC) and cerebellum (CL) were prepared from the adult mouse brain. Cell lysates obtained were then subjected to immunoblot analysis for GLYT1. These experiments were carried out with at least three independent experiments under the same experimental conditions, with similar results.
Effect of GLYT1 inhibition on cell proliferation of the NPC
To evaluate the role of GLYT1 in proliferation of NPC, we examined the effect of GLYT1 inhibitor NFPS on proliferation of the culture of NPCs derived from the hippocampi of embryonic mice (Figure 2). Figure 2a shows phase-contrast images of a neurosphere cultured for 6 DIV in the absence or presence of NFPS at a concentration of 25 µM. The neurospheres cultured in the presence of NFPS were smaller than those cultured in its absence. Treatment with NFPS at a concentration of above 1 μM led to a dose-dependent decrease in the survival of NPCs (Figure 2b). To evaluate the effect of NFPS on the proliferative activity, in addition to cell viability, we assessed BrdU incorporation in the absence or presence of NFPS. NFPS at a concentration of 25 µM led to a significant reduction in BrdU incorporation into the cells.
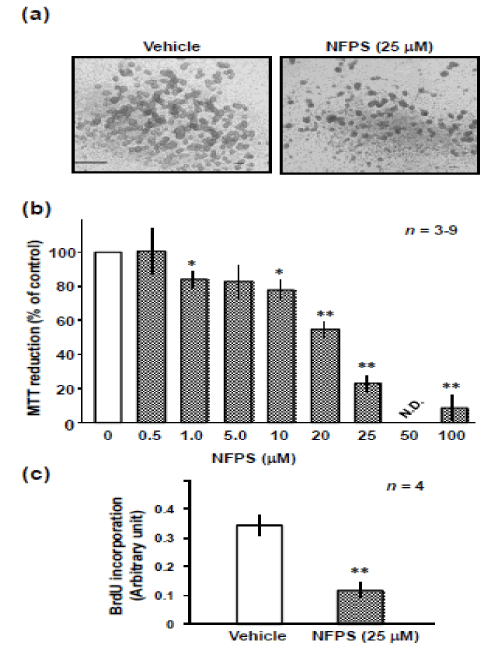
Figure 2: Effect of N-[(3R)-3-([1,1'-Biphenyl]-4-yloxy)-3-(4-fluorophenyl) propyl]-N-methylglycine (NFPS) on growth of the neurosphere. (a) Cells were harvested for subsequent replating and culturing in the growth medium for 6 DIV in the absence or presence of NFPS for determination of cellular MTT reduction activity by MTT assay. Cells were exposed to vehicle, or NFPS at the various concentrations indicated, and then subjected to the MTT assay at 6 DIV. Values are expressed mean ± S.E. from 3 to 9 independent experiments. *P<0.05, **P<0.01, significantly different from each value obtained for cells treated with vehicle alone (concentration = 0). (b) Typical micrographs of the cells in either the presence or absence of NFPS. (c) Cells were harvested for subsequent replating and culturing in the growth medium absence or presence of NFPS for assessment of cell proliferation by a mean of ELISA of 5’-bromo-2’-deoxyuridine (BrdU) at 4 DIV. Values are expressed mean ± S.E. from 4 independent experiments. **P<0.01, significantly different from control obtained for cells treated with vehicle alone (NFPS = 0).
Effect of NFPS on cell survival
To examine whether NFPS is cytotoxic to NPCs, we performed a PI uptake assay, which determines cell damage [14] (Figure 3). Treatment with NFPS for 1 day had no significant effect on the number of PI-positive cells (Figure 3a), suggesting that NFPS did not damage NPCs, at least during the culture period examined.
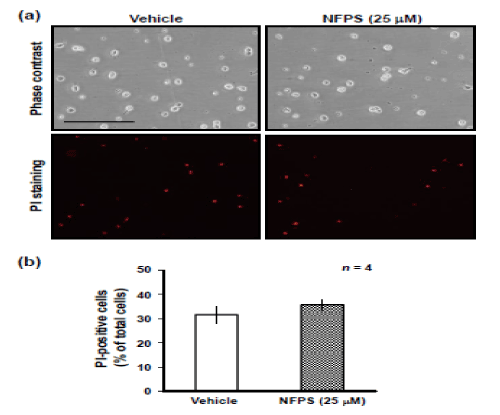
Figure 3: Propidium iodide (PI) uptake assay. The extent and distribution of dying cells were determined after replaced cells for 1 DIV by the addition of PI at a final concentration of 2 mg/mL into the growth medium. The amount cell death was determined by using a fluorescence microscope and camera after 10 min of incubation at 37ºC. (a) Typical micrographs of the cells in either the presence or absence of NFPS. PI staining revealed that NFPS had no significant effect on cell viability in cultured NPCs for 1 DIV. (b) The graph shows proportion of PI (+) cells in total cells. Values are expressed mean ± S.E. from 4 independent experiments.
Discussion
In this study, we demonstrated that GLYT1 positively regulates proliferative activity in the hippocampal NPCs of embryonic mice; this has not been reported previously. This was evidenced by the ability of NFPS to inhibit cell proliferation of the cultures of the embryonic hippocampal NPCs, in which GLYT1 expression was verified. These data support the concept that glycine endogenously activated the proliferation of NPCs in the embryonic mouse hippocampus under physiological conditions. We further suggest that GLYT1 is constitutively activated and thereby positively regulates neuronal development in the embryonic mouse hippocampus under physiological conditions.
The extracellular glycine concentration is uniquely regulated by GLYT1, which is widely expressed in neurons and glia throughout the brain [15,16]. Blocking of GLYT1 is known to enhance the activation of NMDA receptor-dependent synaptic transmission [17] and to reduce neuronal signaling [18,19]. Previous reports have suggested that extracellular glycine is effectively increased by blocking GLYT1 and that this mediates synaptic integration by dual activation of both the glycine receptor and the NMDA receptor [20,21]. Numerous previous studies have shown that proliferation of NPCs is regulated by activation of various receptors and these neurotransmitters; i.e., activation of the GABAA receptor, group I metabotropic glutamate (mGlu) receptor or dopamine D3 receptor enhances proliferation of NPCs [22-24], whereas activation of the NMDA receptor, group III mGlu receptor, or a4b2 nicotinic acetylcholine receptor suppresses proliferative activity [25-28].
It remains somewhat unclear how neurotransmitters regulate proliferation of NPCs. NPCs form no synaptic junction with other neurons. Thus, it has been proposed that neurotransmitters may act directly as paracrine or autocrine factors to NPCs under physiological conditions. Indeed, systemic administration of NMDA decreases proliferation of NPCs in the adult murine hippocampal dentate gyrus [29], whereas administration of a dopamine D3 agonist increased proliferation of NPCs in the adult murine subventricular zone [28]. In the present study, we demonstrated the possibility that glycine enhances proliferation of NPCs. However, there is no direct evidence that exposure of NPCs to glycine enhanced the proliferation under the experimental conditions used in the present study. In fact, glycine had no effect on the proliferation under the same experimental conditions in the present study (data not shown). This may be due to the presence of glycine in the culture medium used. Thus, in the present study, we used a glycine transporter inhibitor. Transporters are responsible for transport of physiological substrates between brain interstitial fluid and intracellular space, and therefore can directly control physiological functions of NPCs. Thus, transporters may be important candidate extracellular/intracellular environment regulator molecules, expressed on the cellular membrane of NPCs. This is exemplified by the present findings regarding the role of GLYT1 in the regulation of NPCs. However, the functional significance of GLYT1 in proliferation of NPCs remains unknown and further studies will be needed to elucidate the mechanism underlying regulation of the glycine-mediated enhancement of proliferation in NPCs.
Conclusion
We here reported the expression of GLYT1 in NPCs derived from the embryonic mouse hippocampus. Uptake of glycine by GLYT1 plays a key role in the cellular proliferation of NPCs in the developing hippocampus. GLYT1-mediated signaling may be considered as a new target for future studies on neurogenesis and neurodevelopment.
Acknowledgments
The authors have no conflicts of interest to declare. This work was supported in part by Grants-in-Aid for scientific research to M.Y. from the Ministry of Education, Culture, Sports, Science, and Technology, Japan.
S.E. from 4 independent experiments.
References
- Reynolds BA, Tetzlaff W, Weiss S (1992) A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J Neurosci 12: 4565-4574. [Crossref]
- Yoneyama M, Kawada K, Gotoh Y, Shiba T, Ogita K (2010) Endogenous reactive oxygen species are essential for proliferation of neural stem/progenitor cells. Neurochem Int 56: 740-746. [Crossref]
- Gage FH (2000) Mammalian neural stem cells. Science 287: 1433-1438. [Crossref]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, et al. (2003) Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 301: 805-809. [Crossref]
- Schaffer DV, Gage FH (2004) Neurogenesis and neuroadaptation. Neuromol Med 5: 1-9. [Crossref]
- Gomeza J, Ohno K, Betz H (2003) Glycine transporter isoforms in the mammalian central nervous system: structures, functions and therapeutic promises. Curr Opin Drug Discov Dev 6: 675-682. [Crossref]
- Aragon C, Lopez-Corcuera B (2005) Glycine transporters: crucial roles of pharmacological interest revealed by gene deletion. Trends Pharmacol Sci 26: 283-286. [Crossref]
- Dohi T, Morita K, Kitayama T, Motoyama N, Morioka N (2009) Glycine transporter inhibitors as a novel drug discovery strategy for neuropathic pain. Pharmacol Ther 123: 54-79. [Crossref]
- Johnson JW, Ascher P (1987) Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature 325: 529-531. [Crossref]
- Gomeza J, Hülsmann S, Ohno K, Eulenburg V, Szöke K, et al. (2003) Inactivation of the glycine transporter 1 gene discloses vital role of glial glycine uptake in glycinergic inhibition. Neuron 40: 785-796. [Crossref]
- Eulenburg V, Armsen W, Betz H, Gomeza J (2005) Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci 30: 325-333. [Crossref]
- Zhang LH, Gong N, Fei D, Xu L, Xu TL (2008) Glycine uptake regulates hippocampal network activity via glycine receptor-mediated tonic inhibition. Neuropsychopharmacol 33: 701-711. [Crossref]
- Yoneyama M, Haebe S, Shiba T, Yamaguchi T, Ogita K (2015) Beneficial effect of cilostazol-mediated neuronal repair following trimethyltin-induced neuronal loss in the dentate gyrus. J Neurosci Res 93: 55-66. [Crossref]
- Umansky SR, Tomei LD (1997) Apoptosis in the heart. Adv Pharmacol 41: 383-407. [Crossref]
- Aragon C, Lopez-Corcuera B (2005) Glycine transporters: crucial roles of pharmacological interest revealed by gene deletion. Trends Pharmacol Sci 26: 283-286. [Crossref]
- Eulenburg V, Armsen W, Betz H, Gomeza J (2005) Glycine transporters: essential regulators of neurotransmission. Trends Biochem Sci 30: 325-333. [Crossref]
- Katsuki H, Watanabe Y, Fujimoto S, Kume T, Akaike A (2007) Contribution of endogenous glycine and d-serine to excitotoxic and ischemic cell death in rat cerebrocortical slice cultures. Life Sci 81: 740-749. [Crossref]
- Martina M, Gorfinkel Y, Halman S, Lowe JA, Periyalwar P, et al. (2004) Glycine transporter type 1 blockade changes NMDA receptor-mediated responses and LTP in hippocampal CA1 pyramidal cells by altering extracellular glycine levels. J Physiol 557: 489-500. [Crossref]
- Chattipakorn SC, McMahon LL (2002) Pharmacological characterization of glycine-gated chloride currents recorded in rat hippocampal slices. J Neurophysiol 87: 1515-1525. [Crossref]
- Chen RQ, Wang SH, Yao W, Wang JJ, Ji F, et al. (2011) Role of glycine receptors in glycine-induced LTD in hippocampal CA1 pyramidal neurons. Neuropsychopharmacol 36: 1948-1958. [Crossref]
- Huang B, Xie Q, Lu X, Qian T, Li S, Z et al. (2016) GLYT1 inhibitor NFPS exerts neuroprotection via GlyR alpha1 subunit in the rat model of transient focal cerebral ischaemia and reperfusion. Cell Physiol Biochem 38: 1952-1962. [Crossref]
- Yoneyama M, Fukui M, Nakamichi N, Kitayama T, Taniura H, et al. (2007) Activation of GABAA receptors facilitates astroglial differentiation induced by ciliary neurotrophic factor in neural progenitors isolated from fetal rat brain. J Neurochem 100: 1667-1679. [Crossref]
- Zhao L, Jiao Q, Chen X, Yang P, Zhao B, et al. (2012) mGluR5 is involved in proliferation of rat neural progenitor cells exposed to hypoxia with activation of mitogen-activated protein kinase signaling pathway. J Neurosci Res 90: 447-460. [Crossref]
- Lao CL, Lu CS, Chen JC (2013) Dopamine D(3) receptor activation promotes neural stem/progenitor cell proliferation through AKT and ERK1/2 pathways and expands type-B and -C cells in adult subventricular zone. Glia 61: 475-489. [Crossref]
- Bunk EC, König HG, Bonner HP, Kirby BP, Prehn JH (2010) NMDA-induced injury of mouse organotypic hippocampal slice cultures triggers delayed neuroblast proliferation in the dentate gyrus: an in vitro model for the study of neural precursor cell proliferation. Brain Res 1359: 22-32. [Crossref]
- Yoneyama M, Nakamichi N, Fukui M, Kitayama T, Georgiev DD, et al. (2008) Promotion of neuronal differentiation through activation of N-methyl-D-aspartate receptors transiently expressed by undifferentiated neural progenitor cells in fetal rat neocortex. J Neurosci Res 86: 2392-2402. [Crossref]
- Nakamichi N, Yoshida K, Ishioka Y, Makanga JO, Fukui M, et al. (2008) Group III metabotropic glutamate receptor activation suppresses self-replication of undifferentiated neocortical progenitor cells. J Neurochem 105: 1996-2012. [Crossref]
- Takarada T, Nakamichi N, Kitajima S, Fukumori R, Nakazato R, et al. (2012) Promoted neuronal differentiation after activation of alpha4/beta2 nicotinic acetylcholine receptors in undifferentiated neural progenitors. PLoS One 7: e46177. [Crossref]
- Yoneyama M, Shiba T, Hasebe S, Ogita K (2011) Adult neurogenesis is regulated by endogenous factors produced during neurodegeneration. J Pharmacol Sci 115: 425-432. [Crossref]



