Peri-implant mucogingival surgery aims at improving esthetic, masticatory function and maintenance care of dental implants. Over the years, various techniques such as Subepithelial Connective Tissue Graft (CTG), Free Gingival Graft (FGG), Rotated Double-pedicle Flap(RDF), Apically Repositioned Flap(ARF) or using Acelluar Dermal Matrix have been introduced.
Mucogingival Graft and Surgery have been employed to increase width and/or thickness of attached gingiva around dental implants; however, these added time and expense for the expecting results and many of them are technically demanding. Some of these techniques require special equipment or materials, complicated procedure and long learning curve. Undesirable complications for unexperienced clinicians might occur. Improvement of this technique is desirable. In order to overcome some of these difficulties, Fu Abutment Stabilization Technique (FAST) is introduced in this article to achieve the predictable results. This method does not need special equipment and materials and can be applied to all implant systems and different stages of implantation. Most importantly, it is simple and minimal-invasive and predictable. This method is expected to be the routine method of increasing attached gingiva at second stage implant surgery in the future.
attached gingiva, dental implant, peri-implant disease, peri-implant surgery, mucogingival surgery, minimal-invasive, second stage implant surgery, apically repositioned flap
Dental implants have become widely accepted and utilized in dental oral rehabilitations by clinicians worldwide over the past two decades [1-6]. Implant treatments have shown endosseous dental implants to be a viable option for the restoration of missing teeth [7,8]. In contrast, based on the Consensus Report of the Sixth European Workshop in Periodontology, Lindhe & Meyle reported an incidence of mucositis of up to 80% and of peri-implantitis between 28% and 56% [9]. The prevention and management of peri-implant disease has become a more and more important issue in implant dentistry. Nowadays, there is no consensus regarding the relationship between the dimensions of keratinized mucosa (KM) and the health of peri-implant tissues, but some clinicians prefer to provide enough keratinized mucosa for long-term maintenance of soft tissue around dental implant [10-13]. Wennström, et al. [14] reported that lacking attached gingiva around implants also resulted in the failure of implants. In the presence of attached gingiva around implants, it can help patients to maintain good oral hygiene, prevent recession of marginal tissue and spread of inflammation by providing tight collar around dental implants. Recent studies showed that mucosal inflammation and plaque accumulation were significantly higher around dental implants with width of KM<2 mm [15]. There was a negative correlation between KM and mucosal recession. An increased width of KM is also associated with lower mean alveolar bone loss [16]. Regarding with thickness of mucosa, Zigdon and Machtei showed that a thick mucosa (≥1 mm) was associated with lesser mucosal recession compared with a thin mucosa (<1 mm) [17]. In the recent treatment of peri-implantitis, if nonsurgical treatment is unsuccessful, surgical approach in reducing the pockets around the affected implants has been advocated in order to enhance self-performed oral hygiene [18]. The presence of adequate attached gingiva and the elimination of peri-implant pockets around dental implants are both important for healthy condition of peri-implant tissues.
Peri-implant mucogingival surgery aims at improving esthetic, masticatory function and self-maintenance care of dental implants. Manipulation of soft tissue around dental implants enables proper peri-implant tissue healing and can result in soft tissue architecture in healthy condition. During the past decade, various techniques such as Subepithelial Connective Tissue Graft (CTG), Free Gingival Graft (FGG), Rotated Double-pedicle Flap (RDF), Apical Repositional Flap (ARF) or the application of Acelluar Dermal Matrix have been introduced, all these methods are categorized as mucogingival surgery by which we are able to manipulate and improve the soft tissue architecture around the implant [19-23].
Subepithelial connective tissue graft (CTG) [24-27]
Subepithelial Connective Tissue Graft (CTG) is commonly harvested from the donor site (e.g. palate). This is used to remedy thin biotype cases and can potentially increase the zone of keratinzed gingiva. It is preferably used in esthetic region in order to maintain the gingival color of the treated area unaltered.
Free gingival graft (FGG) [20]
Free Gingival Graft (FGG) is commonly harvested from the palate or tuberosity to cover around the implant site when there is inadequate keratinized gingiva. It is preferably used in non-esthetic region as the gingival color of the treated area altered [28-30].
Rotated double-pedicle flap (RDF) [22]
In this procedure, two split-thickness keratinized pedicles were dissected from the mesial and distal interproximal tissues near the implant. After rotation, both the pedicles were sutured to each other mid-buccally and the pedicles were rigidly immobilized with sutures. This technique is indicated when the neighboring tissues are in thick biotype with good blood supply. In case of thin mucosa, mucosal recession might happen.
Apically repositioned flap (ARF) [21]
When the keratinized zone around the implant is inadequate, we can apically have positioned flap to mobilize the keratinized zone from the lingual side towards the buccal side of the abutment during second stage implant surgery. It is admittedly the preferably method of widening the keratinized zone in simple procedures.
Acellular dermal matrix [23]
Acellular dermal matrix grafts can replace the connective tissue graft if patients or clinicians do not want a second donor site, but it needs additional expenses for the same results as the connective tissue graft.
All the above methods aim to increase the dimensions of attached gingiva for esthetic, masticatory and maintenance functions and are able to obtain predictable results by experienced clinicians. In this article, we introduce a simplified approach in the stabilization of the apically repositioned flap (ARF) in a predictable way namely, Fu Abutment Stabilization Technique (FAST). We introduce this standard surgical protocol in our department during the past few years, we found that it can help clinicians to stabilize the repositioned flap during ARF procedure in a simple and less traumatic way with predictable outcome.
Fu abutment stabilization technique (FAST)
Preservation or dimensional increase of attached gingiva by mucogingival surgery is considered critical for long-term self-maintenance and success of dental implant. Some surgical techniques in implant surgery will jeopardize the preservation, formation and architecture of attached gingiva; these include punching-tissue technique , scalloping-respective technique and mid-crestal incision techniques without apically repositioned [31]; The above techniques are not recommended when dimensions of attached gingiva are inadequate. Some mucogingival surgical techniques such as FGG and CT graft require complicated procedure and long learning curve [32].
Apically repositioned flap during second stage implant surgery is admittedly the preferable method for widening the keratinized zone. The stabilization of the repositioned flap during the healing process is the key of success for this method. The coronally migration of flap tissue due to unfavorable stabilization might cause undesirable tissue-thickening and pocket around dental implant after healing, that might lead to peri-implant problems. Periodontal dressings that commonly used in natural teeth are not always favorable in stabilizing the repositioned flap around dental implants; especially when there is no neighboring tooth or anchorage near the site. Utilization of sling sutures to stabilize the repositioned flap is also not always favorable, we found that the flaps might unexpectedly migrate coronally towards the abutment during the healing process [21]. Pre-fabricated implant-retained stents for flap stabilization were introduced. Because the stent is seated over the repositioned flap, oral hygiene during healing is difficult to maintain [19].
In this article, we introduce a sophisticated, simplified approach that have been using as standard surgical protocol in our department from the past two years, namely Fu Abutment Stabilization Technique (FAST). We developed and applied this method as the standard surgical protocol for two-staged implant systems with screw-in (healing) abutment types. We found that by using FAST, we might be able to stabilize the repositioned flap at the level of implant-abutment connection in a simple , predictable and minimal-invasive procedure.
Take history
Candidates of stage-one or stage-two surgeries for dental implants are excluded if they exhibit pathological findings or have a history of general diseases or operations. Patients with risk of infection, impaired wound healing, and metabolic diseases is not recommended or contraindicated for operation because it will lead to postoperative infection.
Presurgical evaluation
CT, tomo, pano or periapical X-ray are used for accurate evaluation of osseointegration and position of dental implant(s) before second stage involvement. Check the angulation of implant(s) to see whether it needs any modification of surgical procedure. Determine the position of muco-gingival junction intraorally and measure the adequacy of keratinized gingiva. At least 3mm of KM around dental implant is necessary for oral implant health.
Premedication
2 g Amoxillin or 600 mg 1~2 hours before surgery or Augmentin 375/125 mg twice daily initiated 24 hours before surgery.
In case of penicillin allergy, 600 mg Clindamycin 1~2 hours before surgery.
Mouth rinsing with chlorohexidine 0.12% for one minute before surgery.
Surgical procedure of FAST: (Figures 1a-1e, Figures 2a & 2b)
- Local infiltration anesthesia is used and followed by a remote lingual incision. At least 3mm of KM should be included. Mesial and distal releasing incisions starting from remote lingual are extended towards the buccal vestibule.
- A partial-thickness muco-periosteal flap is dissected and reflected from the lingual towards the buccal side, exposing the cover screw and the alveolar ridge.
- Remove the cover screw and screw down the abutment or gingival former. Complete seating of the abutment is confirmed.
- The flap is repositioned to the buccal side of the alveolar ridge with its coronal flap margin levels with the buccal aspect of the healing abutment.
- Reconfirm that the flap is at the right position on the buccal side of the alveolar ridge. Interrupted sutures are used to stabilize the flap mesio-distally. (Dafilon®, Aesculap AG & Co. KG., Tuttlingen, Germany)
- Unscrew the healing abutment or gingival former, leaving two to three threads unscrewed before complete seating on the implant fixture.
- A single stitch is placed about 2mm from the flap margin proximal to the buccal aspect of the healing abutment, leaving two ends of the stitch: the long end of approx. 40mm and the short end of approx. 20mm. Polytetrafluoroethylene(PTFE) suture material (e.g.Gortex CV-5) is recommended in this procedure because of its elastic and self-cleansing characteristics. The long end of the stich is looped around the healing abutment screw in between the pre-lossened abutment-implant interface for two times.
- Gently pull the long end of the stich towards bucco-apical direction and at the same time screw down the healing abutment until it is tightly seated on the implant head and the coronal flap margin is levelled with the abutment-implant connection. Torque wrench is used to apply torque of 20Ncm.
- Suture the short end and the long end of the stich together.
- Subsidiary muco-periosteal sutures are added to further stabilize the flap so that the mucosa is closely attached to the underlying periosteum.
- A thick sterilized gauze is placed on the buccal side of the flap, another gauze is placed occlusally and then patient is asked to bite for an hour.
Figure 1a. Lingual Incision and partial-thickness muco-periosteal flap elevation towards the buccal side
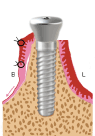
Figure 1b. Put on the healing abutment and then reposition the flap to the buccal aspect of the healing abutment. Interrupted sutures are placed mesio-distally
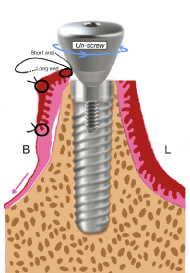
Figure 1c. A single stitch is placed near the flap margin near the buccal aspect of the healing abutment, leaving two ends of stitch: one long end and one short end. Unscrew two to three threads of the healing abutment
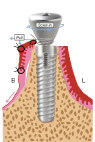
Figure 1d. The long end of the stich is looped around the healing abutment screw in between the implant –abutment interface for two times. Pull the long end of stich towards buccco-apical direction and at the same time screw down the healing abutment
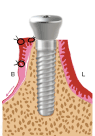
Figure 1e. The healing abutment is tightly seated on the implant head at its final position
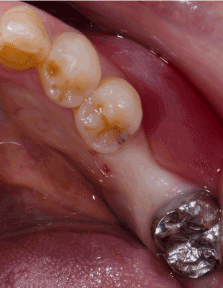
Figure 2a. Oral examination at second stage: buccal concavity and migration of the muco-gingival junction towards the alveolar crest at the implant site is found
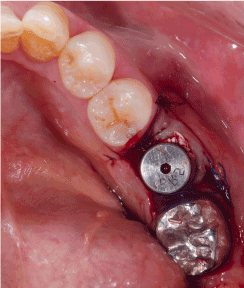
Figure 2b. FAST is applied
Medication
Pain control: Ibuprofen 600 mg one dose (or 400mg Ibuprofen at 2~3intervals per day)
Infection control: Amoxicillin/Clavulanic acid (Augmentin) 875 mg/125 mg bid for 7 days
Mouth rinsing’s are performed with chlorhexidine 0.12% after meals (three times a day)
Suture released: Sutures are removed 14 days after surgery. The healing abutment is removed temporarily for stich removal and then put back in place for further healing.
Final healing: The healing abutment should be in place for 3 more weeks after suture release in order to have complete, good tissue healing.
Final impression: Either conventional impression method or open/closed tray transfer methods can be done. In case of conventional method, the healing abutment is replaced by the final abutment for final impression. In case of open or closed tray transfer method, the healing abutment is replaced by the impression post or coping for final impression.
Implant protheses delivery: Check fitness of margin and then check occlusion to avoid overloading and interferences in excusive movements.
Follow-up: The patient is observed every 6 months, till 2 years. After this, routine oral examination per year.
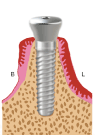
Figure 1f. Sutures are removed 14 days after surgery. The healing abutment is removed temporarily for stich removal and then put back in place
Figure 1g. Healing abutment is replaced by the final abutment for impression
Figure 1h. Delivery of final implant prosthesis. Attached gingival is preserved and deep pocket does not exist
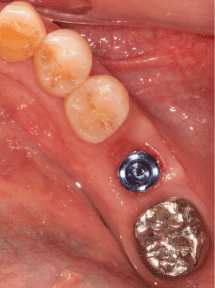
Figure 2c. Widened attached gingiva and ridge profile are obtained two weeks after release of sutures
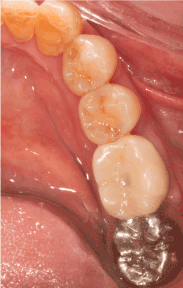
Figure 2d. Delivery of prosthesis with good emergence profile and soft tissue biotype around dental implant
An adequate amount of keratinized mucosa (KM) firmly attached to the underlying periosteum so called attached gingiva has been cited as a goal in implant maintenance. Friedman stated that “inadequate” zone of attached gingiva would facilitate subgingival plaque formation because of improper pocket seal resulting from the movability of the marginal tissue. The absence of keratinized mucosa increases the susceptibility of peri-implant lesions and plaque-induced destruction [33]. Goldman and Cohen postulated that a tight tissue barrier was created like natural dentition, which correlated well with the tissue barrier concept [34]. Mehdi Adibrad, et al. reported that there was a significant influence of the width of keratinized mucosa on the health of the peri-implant tissues. The absence of adequate keratinized mucosa around implants supporting overdentures was associated with higher plaque accumulation, gingival inflammation, bleeding on probing, and mucosal recession; In addition, mainly because of the thickness of mucosa obtained by soft tissue augmentation, the patient was able to maintain the soft tissue around the implant with lesser plaque formation [35]. It is the general belief that wide and thick KM are of special importance in the maintenance of implant health, while narrow and thin KM may lead to greater mucosal recession.
Some surgical techniques of second stage implant surgery will jeopardize the preservation, formation and architecture of attached gingiva. When dimensions of attached gingiva are inadequate, surgical techniques such as punching-tissue, scalloping resection and mid-crestal incision techniques are not recommended. A certain amount of keratinized mucosa will be sacrificed during these procedures. If we use mid-crestal incision and screw-in the healing abutment (gingival former) without apically repositioning the buccal and lingual flap tissue, it will result in deep pockets around implant; if we do apically repositioned flap without good stabilization of the mobilized flap, it will also result in deep pockets around implant. In both cases, they might lead to mucositis and periimplantitis.
In recent literature, the procedure most indicated to increase the width of attached gingiva seems to be the free gingival graft (FGG) or connective tissue graft (CTG). FGG or CTG techniques are indicated when apically repositioned flap cannot satisfy the need of attached gingiva at second stage surgery [20,28-30]. However, they have some disadvantages, such as the need for a second donor site; two surgical sites will increase time of surgery and increase pain after surgery. FGG and CTG require complicated procedure and long learning curve [32]. With the exception of the aforementioned conditions, apically repositioned flap during second stage implant surgery is admittedly the preferable method of widening the keratinized zone around implant in simple procedures. In this procedure, stabilization of the repositioned flap will affect the final outcome. Without good stabilization, the apically repositioned flap will relapse coronally. The flabby tissue unattached to the underlying periosteum might undergo shrinkage due to lack of periosteal blood supply and/or it might develop undesirable pockets around dental implant. Shrinkage of the apically repositioned flap will reduce the dimensions of attached gingiva, while the development of undesirable pockets may favor plaque accumulation which might cause mucositis and perimplantitis. Periodontal dressings are not favorable in stabilizing the repositioned flap when there is no neighboring tooth for anchorage near the implant site. Some clinicians suggested to use sling suture to fix the repositioned flap [21], but there is also tendency of flap-relapse coronally during the healing process. Other clinicians use specially designed abutment-retained stents to apply pressure over the repositioned flap in order to stabilize the flap from coronally relapsing, but the oral hygiene during healing is more difficult to maintain [19]. It will potentially affect blood supply and decrease pre-existing keratinized mucosa, causing gingival recession.
In our clinical experience, there is usually sufficient amount (>3mm) of keratinized mucosa for repositioning from the lingual side of the alveolar ridge. However, if the keratinized mucosa is not adequately immobilized to the underlying bone at the buccal ridge of the implant, it will migrate coronally and will cause reduction of the attached gingiva on the buccal ridge. If the zone of keratinized mucosa is limited, it will not only minimize the function as tight tissue barrier, but it will also form pockets around the implant and become more susceptible to peri-implant lesions and plaque-induced destruction. Using the FAST procedure that we introduced in this article, the stich that placed near the flap margin proximal to the buccal aspect of the (healing) abutment is looped around the healing abutment screw in between the abutment-implant interface. The coronal flap margin is accurately stabilized three-dimensionally by the stich which is finally by the healing abutment. This eliminates the risk of migration of the flap coronally. Because the zone of keratinized gingiva repositioned from the lingual side is ultimately preserved and attached to the underlying bone at the buccal side, a tight peri-implant mucosal barrier around the dental implant can be formed; Long-term maintenance and success of the dental implant become predictable.
It is imperative that clinicians should know the important role of attached ginigva in long-term maintenance and success of the dental implant. Fu Abutment Stabilization Technique (FAST) protocol is simple and effortless in preserving adequate amount of attached gingiva around dental implants. It has the following advantages:
a. It provides good stability of the repositioned flap tissue to the underlying bone.
b. It maximizes keratinized gingiva attachment to the underlying bone.
c. It preserves the maximum amount of keratinized gingiva around implant.
d. It minimizes undesirable deep pockets around the implant.
e. It stabilizes the repositioned flap apically at the abutment-implant connection level and prevent coronal migration of the flap, thus enhances emergence profile of the final prosthesis.
f . It provides good blood supply for tissue healing because the keratinized mucosa is stabilize and attached over the periosteum.
h. It can be applied to most of the two-staged implant systems.
i. It does not need extra equipment or tool.
j. The procedure is simple, easy to learn and manipulate. Patients feel comfortable during the entire process.
To further establish the efficacy of this surgical protocol, large amount clinical evidence and long term follow-up will be required.
The author claims to have no financial interest, directly or indirectly, in any entity that is commercially related to the products mentioned in this article.
The authors would like to acknowledge Mr. Kenneth Fu Kwan-loun (atomic8733@gmail.com) for his technical support in graphic illustrations.
- Mono-filament silk suture(Dafilon®, Aesculap AG & Co. KG., Tuttlingen, Germany)
- Gortex suture(GORE-TEX® Suture, Gore Medical, Flagstaff, Arizona, USA)
- Dental implants ( BioHorizons Implant Systems Inc., Birmingham. Alabama)
- Astrand P, Ahlqvist J, Gunne J, Nilson H (2008) Implant treatment of patients with edentulous jaws: a 20-year follow-up. Clin Implant Dent Relat Res 10: 207-217. [Crossref]
- Lekholm U, Gröndahl K, Jemt T (2006) Outcome of oral implant treatment in partially edentulous jaws followed 20 years in clinical function. Clin Implant Dent Relat Res 8: 178-186. [Crossref]
- Adell R, Lekholm U, Rockler B, Brånemark PI (1981) A 15-year study of osseointegrated implants in the treatment of the edentulous jaw. Int J Oral Surg 10: 387-416. [Crossref]
- Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T (1990) Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofacial Implants 5: 347-359. [Crossref]
- Albrektsson T, Dahl E, Enbom L, Engevall S, Engquist B, et al. (1988) Osseointegrated oral implants. A Swedish multicenter study of 8139 consecutively inserted Nobelpharma implants. J Periodontol 59: 287-296. [Crossref]
- Engquist B, Bergendal T, Kallus T, Linden U (1998) A retrospective multicenter evaluation of osseointegrated implants supporting overdentures. Int J Oral Maxillofac Implants 3: 129-134. [Crossref]
- Misch CE, Misch-Dietsh F, Silc J, Barboza E, Cianciola LJ, et al. (2008) Posterior implant single-tooth replacement and status of adjacent teeth during a 10-year period: a retrospective report. J Periodontol 79: 2378-2382. [Crossref]
- Kronström M, Widbom C, Soderfeldt B (2006) Patient evaluation after treatment with maxillary implant-supported overdentures. Clin Implant Dent Relat Res 8: 39-43. [Crossref]
- Lindhe J, Meyle J; Group D of European Workshop on Periodontology (2008) Peri-implant diseases: Consensus report of the sixth European workshop on periodontology. J Clin Periodontol 35: 282-285. [Crossref]
- Brito C, Tenenbaum HC, Wong BK, Schmitt C, Nogueira-Filho G (2014) Is keratinized mucosa indispensable to maintain peri-implant health? A systematic review of the literature. J Biomed Mater Res B Appl Biomater 102: 643-650. [Crossref]
- Malo P, Rigolizzo M, Nobre M, Lopes A, Agliardi E (2013) Clinical outcomes in the presence and absence of keratinized mucosa in mandibular guided implant surgeries: a pilot study with a proposal for the modification of the technique. Quintessence Int 44: 149-157. [Crossref]
- Wennstrom JL, Derks J (2012) Is there a need for keratinized mucosa around implants to maintain health and tissue stability? Clin Oral Implants Res 23: 136-146. [Crossref]
- Andreasen J, Kristerson L, Nilson H, Dahlin K, Schwatz O, Palacci P, et al. (1994) Andreasen JO, Andreasen FM. Textbook and Color Atlas of Traumatic Injuries to the teeth. 3rd ed. Ch. 20. Copenhagen: Munksgaard; 1994. Implants in the anterior region.
- Wennström JL, Bengazi F, Lekholm U (1994) The influence of the masticatory mucosa on the peri-implant soft tissue condition. Clin Oral Implants Res 5: 1-8. [Crossref]
- Chung DM, Oh TJ, Shotwell JL, Misch CE, Wang HL (2006) Significance of keratinized mucosa in maintenance of dental implants with different surfaces. J Periodontol 77: 1410-1420. [Crossref]
- Artzi Z, Carmeli G, Kozlovsky A (2006) A distinguishable observation between survival and success rate outcome of hydroxyapatite-coated implants in 5-10 years in function. Clin Oral Implants Res 17: 85-93. [Crossref]
- Bouri A Jr, Bissada N, Al-Zahrani MS, Faddoul F, Nouneh I (2008) Width of keratinized gingiva and the health status of the supporting tissues around dental implants. Int J Oral Maxillofac Implants 23: 323-326. [Crossref]
- Smeets R, Henningsen A, Jung O, Heiland M, Hammächer C, et al. (2014) Definition, etiology, prevention and treatment of peri-implantitis--a review. Head Face Med 10: 34. [Crossref]
- Park JC, Yang KB, Choi Y, Kim YT, Jung UW, et al. (2010) A simple approach to preserve keratinized mucosa around implants using a pre-fabricated implant-retained stent: a report of two cases. J Periodontal Implant Sci 40: 194-200. [Crossref]
- Kaufmann R, Bassetti R, Mericske-Stern R, Enkling N (2014) Broadening the keratinized periimplant mucosa at the time the implant reopening. Swiss Dent J 124: 1315-1331.
- Reddy AA, Kumar PA, Sailaja S, Chakravarthy Y, Chandra RV (2015) Concomitant correction of a soft-tissue fenestration with keratinised tissue augmentation by using a rotated double-pedicle flap during second-stage implant surgery- a case report. J Clin Diagn Res 9: ZD16-9. [Crossref]
- Liu C, Su Y, Tan B, Ma P, Wu G (2014) Reconstruction of attached soft tissue around dental implants by a celluar dermal matrix grafts and resin splint. Int J Clin Exp Med 7: 4666-4676. [Crossref]
- Fu JH, Su CY, Wang HL (2012) Esthetic soft tissue management for teeth and implants. J Evid Based Dent Pract 12: 129-142. [Crossref]
- Esposito M, Maghaireh H, Grusovin MG, Ziounas I, Worthington HV (2012) Soft tissue management for dental implants: what are the most effective techniques? A cochrane systematic review. Eur J Oral Implantol 5: 221-238. [Crossref]
- Scharf DR, Tarnow DP (1992) Modified roll technique for localized alveolar ridge augmentation. Int J Periodontics Restorative Dent 12: 415-425. [Crossref]
- Al-Hamdan KS (2011) Esthetic soft tissue ridge augmentation around dental implant: Case report. Saudi Dent J 23: 205-209. [Crossref]
- Kan JY, Rungcharassaeng K, Umezu K, Kois JC (2003) Dimensions of peri-implant mucosa: an evaluation of maxillary anterior single implants in humans. J Periodontol 74: 557-562. [Crossref]
- Hansbrough JF, Franco ES (1998) Skin replacements: Wound healing: State of the art. Clin Plast Surg 10: 407-422.
- Haghighati F, Mousavi M, Moslemi N, Kebria MM, Golestan B (2009) A comparative study of two root-coverage techniques with regard to interdental papilla dimension as a prognostic factor. Int J Periodontics Restorative Dent 29: 179-189. [Crossref]
- Adell R, Lekholm U, Branemark PI. Branemark PI, Zarb GA, Albertsson T (1985) Tissue integrated prosthesis: Osseointegration in clinical dentistry. 9th ed. Chicago: Quintessence; 1985. Surigcal procedures; pp. 211-32.
- Moy PK, Weinlaender M, Kenney EB (1989) Soft-tissue modifications of surgical techniques for placement and uncovering of osseointegrated implants. Dent Clin North Am 33: 665-681. [Crossref]
- Freidman N. Mucogingival surgery. The Apically repositioned flap. Journal of periodontology 33: 328-340.
- Goldman H (1979) Periodontal therapy. 6th ed. St. Louis: CVMosby; 5.
- Adibrad M, Shahabuei M, Sahabi M (2009) Significance of the width of keratinized mucosa on the health status of the supporting tissue around implants supporting overdentures. J Oral Implantol 35: 232-237. [Crossref]












