Abstract
Citrus fruits are one of the most important sources of income in many countries and are widely cultivated in the Mediterranean area. During postharvest packing, storage and transportation, citrus fruits can be infestated by a variety of diseases such as mold infection. Thus, the control of postharvest disease is essential for the quality and shelf-life of citrus fruits.
In this present study, the DiaClean® water technology which is based on the electrolysis of a 1.25% NaHCO3 solution with boron-doped diamond electrodes as an alternative to the treatment of citrus fruits with fungicides, was used for fruit preservation. Aliquots of the total mixtures of compounds generated at different stages of the process were used to investigate their cytotoxic effects as an indicator for the evaluation of human health and environmental risks. Cultured connective tissue fibroblasts (cell line L-929) were used for the assays and cell vitality was checked by measurement of the enzymatic activity of mitochondrial dehydrogenases within the cells.
The results clearly demonstrate that the use of electrolyzed NaHCO3 in the preservation of citrus fruits is very effective in the acute stage of electrolysis; the occurrence of antimicrobial and cytotoxic compounds is limited to the liquid stages of the process. After air-drying, no cytotoxic effect was observed which might be harmful to human health and the environment.
Key words
citrus fruit, preservation, storage, reactive oxygen species, cytotoxicity, cell culture
Abbreviations
BDD: Boron-doped diamond electrodes, ROS: reactive oxygen species
Introduction
Citrus fruits are one of the most important sources of income in many countries and are widely cultivated in the Mediterranean area. During postharvest packing, storage and transportation, which may take several weeks, citrus fruits can be infestated by a variety of diseases. Among these, the disease caused by a green mold Penicillium digitatum is one of the most serious ones and might cause up to 90% of product losses [1]. Thus, the control of postharvest disease is essential for the quality and shelf-life of citrus fruits.
The use of various fungicides and herbicides such as imazalil (fungicide), thiabendazole (fungicide and herbicide), pyrimethanil (fungicide) and others has minimized postharvest infestation [2], but the extensive application has led to resistant strains, which decrease their effectiveness. In addition, human health and environmental risks are associated with the use of such fungicides [3]. As a matter of fact, alternative strategies which combine effectiveness and low health and environmental risks have been developed during the last years.
One of the very promising technologies for this problem seems to be the use of electrolyzed water which has been tested successfully in food industrial processes [4] and against postharvest decay of fresh fruits and vegetables [5-7]. Thus, the use of Boron Doped Diamond (BDD) electrodes for electrolysis of citrus fruit wash water proved to be effective and could be increased by the addition of NaHCO3 to the water prior to electrolysis [8-10]. The technology is based on the idea of using diamond as an electrode material in electrochemistry. Especially BDD electrodes have shown outstanding electrochemical properties in the oxidation of organic and inorganic compounds [11]. The reactivity can be mainly associated with the production of hydroxyl radicals [12] or superoxide anion radicals [10]. However, the detailed mode of action is currently not well understood as well as its possible potential to generate long-lasting compounds which might be harmful for human health and environment.
In this present study, the DiaClean® water technology which is based on the electrolysis of a NaHCO3 solution with BDD electrodes, was used for citrus fruit preservation. Aliquots of the total mixtures of compounds generated at different stages of the preservation process were used to investigate their effect on vitality of cultured connective tissue fibroblasts, and thus, to evaluate possible health and environmental risks. To our knowledge, the cytotoxicity of the subsequent process stages has not been examined before.
Materials and methods
Preparation of electrolyzed water according to DiaClean® technology
2.5 L of supply water (15°C) were poured into the reservoir of the DiaClean® Kit equipment (WaterDiam France SAS; Franken, France) and electrolyzed with a current flow of 4.0 A, and a volumetric flow rate of 200 L/h. The voltage was 25 V during electrolysis. The water was cooled during electrolysis by a cooling coil with circulating cold water to avoid a temperature increase above 20°C. After 30 min a total charge of 0.8 Ah/L was delivered to the water which is similar as described in the report of Fallanaj et al. [10]. An aliquot of the electrolyzed water was filtered sterile with a 0.45 µm porous membrane filters and added to the cell cultures.
Preparation of electrolyzed 1.25% aqeous NaHCO3 solution
2.5 L of supply water containing 1.25% (w/v) NaHCO3 solution (31.25 g NaHCO3 dissolved in 2.5 L of supply water) were subjected to electrolysis by the DiaClean® Kit equipment as described above with a current flow of 4.0 A and a volumetric flow rate of 200 L/h. The voltage was 8 V during electrolysis. After 30 min a total charge of 0.8 Ah/L was delivered to the NaHCO3 solution which was then filtered sterile and added to the cell cultures.
Overview of all experiments with electrolyzed water and NaHCO3 solution
Based on the preparations of electrolyzed water and NaHCO3 solution as described above, we conducted the following experiments to come to a closer understanding at which stages of the process a cytotoxic effect might occur:
1 Cytotoxicity of supply water ± electrolysis
1.1 Supply water
1.2 Supply water electrolyzed with 0.8 Ah/L directly after electrolysis
1.3 Supply water electrolyzed with 0.8 Ah/L and after storage for 15 min at room temperature
2 Cytotoxicity of 1.25% NaHCO3 solution ± electrolysis
2.1 1.25% NaHCO3 solution before electrolysis
2.2 1.25% NaHCO3 solution directly after electrolysis with 0.8 Ah/L and after storage for 15 min, 2 h and 5 days at room temperature
2.3 1.25% NaHCO3 solution directly after electrolysis with varying total charges of 0.05 - 0.1 - 0.2 - 0.4 - 0.6 - 0.8 Ah/L
3 Cytotoxicity of citrus fruits after treatment with electrolyzed 1.25% NaHCO3 solution
Untreated/unpreserved BIO citrus fruits (oranges, lemons and grapefruits – fruits were selected for absence of any disease or surface wounding) were carefully prewashed with supply water of 40 °C, dipped for 45 to 60 seconds in freshly prepared electrolyzed NaHCO3 (total charge of 0.8 Ah/L), air-dried at normal daylight for 24 h and stored at room temperature for another 24 h. Then, fruits were washed in 25 ml of supply water (20 °C) for each fruit and the sterilized washing solutions were used for the cytotoxicity tests.
Cell culture and culture conditions
L-929 (mouse connective tissue fibroblasts; ACC 173; Leibniz-Institut - Deutsche Sammlung von Mikroorganismen und Zellkulturen; Braunschweig, Germany); internal passage 93-97; recommended for assessment of in vitro cytotoxicity according to EN ISO 10993-5: 2009. Cells were routinely cultivated as mass cultures in an incubator at 37 °C with a moist atmosphere of 5% CO2 and 95% air. Culture medium was RPMI 1640 supplemented with 10% fetal bovine serum and 100 Units/ml of penicillin and 100 µg/ml of streptomycin.
Cytotoxicity assay
The investigations were performed according to EN ISO 10993-5: 2009 (tests for in vitro cytotoxicity) with some modifications.
L-929 cells were taken from 80 to 90% confluent mass cultures and seeded into 96-well plates at a density of 5,000 cells/well in 200 µL of culture medium. 24-48 hours after seeding, cells were exposed to increasing concentrations (up to 50%) of sterile supply water as solvent control and the solutions from the various experimental designs as described in detail. After 3 days of continuous exposure, cell cultures were examined morphologically and cell vitality was measured by the activity of mitochondrial dehydrogenases within the cells using XTT (Xenometrix AG; Allschwil, Switzerland).
XTT is the sodium salt of 2,3-bis[2-methoxy-4-nitro-5-sulfopheny]-2H-tetrazolium-5-carboxyanilide and has a yellowish colour. Mitochondrial dehydrogenases of viable cells cleave the tetrazolium ring of XTT yielding orange formazan crystals which are soluble in aqueous solutions. The intensity of the resulting orange solution correlates directly with cell vitality and metabolic activity [13,14].
For measurement of cell vitality after exposure, culture medium ± added solutions were removed by aspiration and 200 µL/well of fresh culture medium containing 10% XTT were added. After another 120 minutes of incubation at 37 °C with the tetrazolium dye, optical density per well was determined at 450 nm and 690 nm as a difference measurement using a double-wavelength BioTEK Elx 808 ELISA reader. Optical densities at different extract dilutions was compared with corresponding reagent controls and relative cell vitality was calculated as mean value ± standard deviation. All experiments were done either in triplicate or quadruplicate. Statistical analysis was done by using the two-tailed Wilcoxon-Mann-Whitney U-test.
Results and discussion
The addition of supply water caused a concentration dependent reduction in cell vitality of connective tissue fibroblasts after a continuous exposure time of 3 days due to the decreased osmolarity of the culture medium (Figure 1). Therefore, further experiments were only conducted with a maximum of added water of 50 vol% and all data were expressed as relative values in comparison to untreated controls containing the same amount of supply water without any treatment.
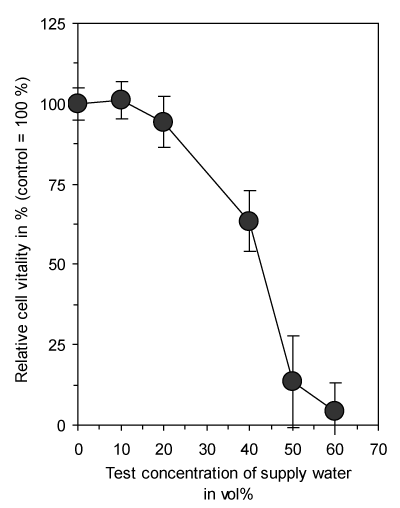
Figure 1. Dose-dependent reduction of vitality of cultured connective tissue fibroblasts (L-929) with increasing concentrations of supply water present in the culture medium by reduction of osmolarity. Exposure time was 3 days. Data represent mean value ± standard deviation (n = 3).
Electrolyzed water with a total charge of 0.8 Ah/L had no effect on the vitality of cultured fibroblasts (Figure 2) neither directly after electrolysis nor after 15 min of storage at room temperature indicating that no cytotoxic compounds have been generated which would be able to reduce the incidence of green mold on the surface of unpreserved citrus fruits. In previous investigations, electrolyzed water has been tested for its use in industrial food processes [4] and against postharvest decay of fresh fruits and vegetables [5-7]. However, the acidification or addition of various organic and inorganic salts to the water during electrolysis enhanced its antimicrobial efficacy. Especially the introduction of NaHCO3 proved to be very effective in the preservation of citrus fruits [15] and it was shown to be a potent inhibitor of Penicillium rots [8,9].
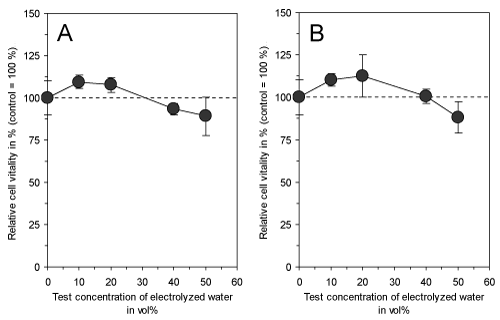
Figure 2. Effect of electrolyzed water (total charge of 0.8 Ah/L) directly (A) and after 15 min of storage at room temperature (B) on vitality of cultured connective tissue fibroblasts (L-929) after 3 days of continuous exposure. Both experimental setups do not show a significant difference between treated cells and corresponding controls. Data represent mean value ± standard deviation (n = 3).
In accordance to the effectiveness of electrolyzed NaHCO3 as demonstrated by Fallanaj et al. [8-10] for Penicillium inhibition, were the data on cytotoxicity obtained with 1.25% NaHCO3 in supply water as presented here. While the exposure of cultured connective tissue fibroblasts to 1.25% NaHCO3 in supply water prior to electrolysis had no influence on cell vitality after 3 days (Figure 3A), the electrolyzed NaHCO3 (total charge of 0.8 Ah/L) directly after electrolysis and after a time period up to 5 days of storage resulted in a marked cytotoxic effect (Figures 3B-3F). However, the cytotoxicity slowly decreased with storage time, but was still statistically significant at test concentrations > 10 vol% after 5 days of storage. This indicates that electrolysis of NaHCO3 resulted in the generation of cytotoxic compounds which seem to be responsible for the antimicrobial efficacy of this process in citrus fruit preservation. In order to test whether a variation of total charge applied to 1.25% NaHCO3 in supply water might influence the observed cytotoxic effect, a total charge ranging from 0.05 Ah/L to 0.8 Ah/L was used and cytotoxicity was examined. As shown in Figure 4, electrolysis of NaHCO3 generated cytotoxic compounds in a charge-dependent manner.
Both results clearly indicate that electrolysis of 1.25% NaHCO3 resulted in the generation of cytotoxic and antimicrobial compounds. Although the process stages are not completely understood, there are some reports on the generated compounds. Jeong et al. [16] reported that the electrochemical production of reactive oxygen species (ROS) could be responsible for microbial inhibition. Moreover, the BDD electrode itself might also produce ROS including superoxide anion radical and hydrogen peroxide [10,17].
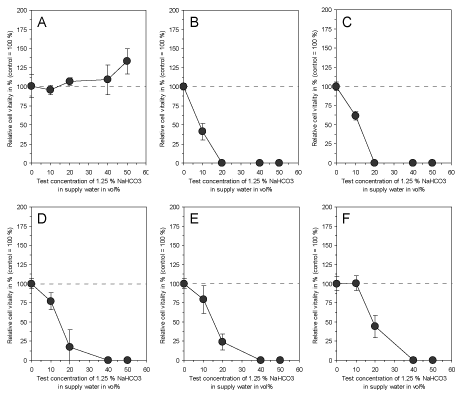
Figure 3. Effect of 1.25 % NaHCO3 dissolved in supply water before electrolysis (A), directly after electrolysis with a total charge of 0.8 Ah/L (B) and after a storage at room temperature for 15 min (C), 2 h (D), 12 h (E) and 5 days (F) on vitality of cultured connective tissue fibroblasts (L-929) after 3 days of continuous exposure. Note that the storage time slowly reduces the cytotoxicity of the electrolyzed NaHCO3, but is still prominent at concentrations higher than 10 vol% after 5 days. Data represent mean value ± standard deviation (n = 3).
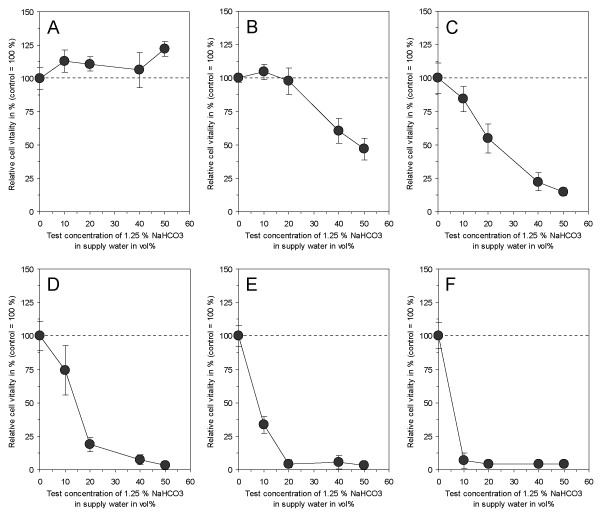
Figure 4. Effect of 1.25 % NaHCO3 dissolved in supply water before electrolysis (A) and directly after electrolysis with a total charge of 0.05 Ah/L (B), 0.1 Ah/L (C), 0.2 Ah/L (D), 0.4 Ah/L (E) and 0.6 Ah/L (F) on vitality of cultured connective tissue fibroblasts (L-929) after 3 days of continuous exposure. Note that electrolysis of NaHCO3 obviously generates cytotoxic compounds in a charge-dependent manner. Data represent mean value ± standard deviation (n = 3).
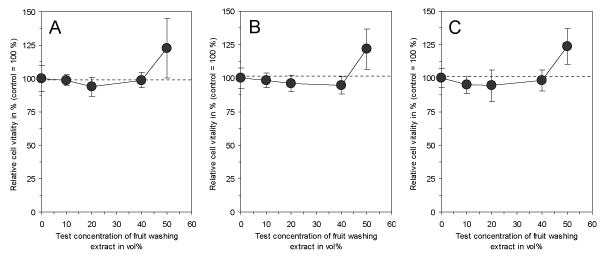
In the final experimental series of this study, it was tested whether the application of freshly prepared electrolyzed NaHCO3 to unpreserved fruits with subsequent air-drying and storage might also result in cytotoxic and harmful compounds. The air-dried surface of oranges, lemons and grapefruits was somewhat powdery and could be easily washed off and collected. This washing extract showed no longer a cytotoxic effect in the test assays at all concentrations in comparison to the controls (Figure 5). This suggests that the primarily generated cytotoxic compounds of the electrolyzed NaHCO3 as depicted in Figures 3 and 4, which are necessary to reduce the incidence of mold, are no longer present after air-drying of the surface. In addition, the experiments also demonstrated an increased cell vitality for all washing extracts at the highest test concentration, probably giving an indication that the treated fruits might be preserved in a way which does not only enhance their shelf-life, but might also result in an improved fruit quality.
2021 Copyright OAT. All rights reserv
Figure 5. Effect of the surface washing extracts of orange (A), lemon (B) and grapefruit (C) on vitality of cultured connective tissue fibroblasts (L-929) after a continuous exposure time of 3 days. Note that no one of the washing extracts is cytotoxic and does not reduce cell vitality in comparison to untreated controls. Moreover, the highest test concentration increases cell vitality in all extracts. Data represent mean value ± standard deviation (n = 4).
Conclusion
In conclusion, the use of electrolyzed NaHCO3 in the preservation of citrus fruits is very effective in the acute stages of electrolysis; the occurrence of antimicrobial and cytotoxic compounds is limited to the liquid stage of the process. After air-drying, no cytotoxic effect was observed which might be harmful to human health and the environment.
References
- Macarisin D, Cohen L, Eick A (2007) Penicillium digitatum suppresses production of hydrogen peroxide in host tissue during infection of citrus fruit. Phytopathology 97: 1491-1500. [Crossref]
- Kanetis L, Förster H, Adaskaveg JE (2007) Comparative efficacy of the new postharvest fungicides azoxystrobin, fludioxonil, and pyrimethanil for managing citrus green mold. Plant Dis 91: 1502-1511.
- Şişman T, Türkez H (2010) Toxicologic evaluation of imazalil with particular reference to genotoxic and teratogenic potentials. Toxicol Industrial Health 26: 641-648. [Crossref]
- Kim C, Hung YC, Brachett RE (2000) Roles of oxidation-reduction potential in electrolyzed oxidizing and chemically modified water for inactivation of food related pathogens. J Food Prot 63: 19-24. [Crossref]
- Al-Haq MI, Seo Y, Oshita S, Kawagoe Y (2002) Disinfection effect of electrolyzed oxidizing water on suppressing fruit rot of pear caused by Botryosphaeria berengeriana. Food Res Int 35: 657-664.
- Cui X, Shang Y, Shi Z, Xin H, Cao W (2009) Physicochemical properties and bactericidal efficiency of neutral and acidic electrolyzed water under different storage conditions. J Food Eng 91: 582-586.
- Guentzel JL, Lam KL, Callan MA, Emmons SA, Dunham VL (2010) Postharvest management of gray mould and brown rot on surfaces of peaches and grapes using electrolyzed oxidizing water. Int J Food Microbiol 143: 54-60. [Crossref]
- Fallanaj F, Sanzani SM, Zavanella C, Ippolito A (2013) Salt addition improves the control of citrus postharvest diseases using electrolysis with conductive diamond electrodes. J Plant Pathol 95: 373-383.
- Fallanaj F, Sanzani SM, Youssef K, Zavanella C, Salerno MG, Ippolito A (2015) A new perspective in controlling postharvest citrus rots: the use of electrolyzed water. Acta Hort 1065: 1599-1606.
- Fallanaj F, Ippolito A, Ligorio A, Garganese F, Zavanella C, Sanzani SM (2016) Electrolyzed sodium bicarbonate inhibits Penicillium digitatum and induces defence responses against green mould in citrus fruit. Postharvest Biol Technol 115: 18-29.
- Gandini D, Mahé E, Michaud PA, Haenni W, Perret A, Comninellis C (2000) Oxidation of carboxylic acids at boron-doped diamond electrodes for wastewater treatment. J Appl Electrochem 12: 1345-1350.
- Marselli B, Garcia-Gomez J, Michaud PA, Rodrigo MA, Comninellis C (2003) Electrogeneration of hydroxyl radicals on boron-doped diamond electrodes. J Electrochem Soc 150: D79-D83.
- Roehm NW, Rodgers GH, Hatfield SM, Glasebrook AL (1991) An improved colorimetric assay for cell proliferation and viability utilizing the tetrazolium salt XTT. J lmmunol Meth 142: 257-265. [Crossref]
- Brosin A, Wolf V, Mattheus A, Heise H (1997) Use of XTT-assay to assess the cytotoxicity of different surfactants and metal salts in human keratinocytes (HaCaT). A feasible method for in vitro testing of skin irritants. Acta Dermato-venereologica 77: 26-28. [Crossref]
- Smilanick JL, Margosan DA, Mlikota F, Usall J, Michael IF (1999) Control of citrus green mold by carbonate and bicarbonate salts and the influence of commercial postharvest practices on their efficacy. Plant Dis 83: 139-145.
- Jeong J, Kim C, Yoon J (2009) The effect of electrode material on the generation of oxidants and microbial inactivation in the electrochemical disinfection processes. Water Res 43: 895-901.
- Hao J, Qiu S, Li H, Chen T, Liu H, Li L (2012) Roles of hydroxyl radicals in electrolyzed oxidizing water (EOW) for the inactivation of E. coli. Int J Food Microbiol 155: 99-104. [crossref]





 2021 Copyright OAT. All rights reserv
2021 Copyright OAT. All rights reserv