Abstract
The Ebola outbreak 2013-2015 created an urgent need for humanitarian response. Public Health England (PHE), in partnership with the Ministry of Defence (MoD) and Defence Science and Technology Laboratory (DSTL), were tasked by the UK Government (through the Department for International Development (DfID)) to provide Ebola Virus Disease (EVD) diagnostic laboratories. These diagnostic laboratories supported the Ebola Treatment Units (ETU) being established in Sierra Leone. PHE operated arguably the largest diagnostic facilities in Sierra Leone by one unilateral donor: operating 3 laboratories (co-located with ETUs), each processing up to 200 samples a day. During the time of operation (October 2014 to December 2015) over 400 civilian UK staff on rotation were deployed in these laboratories, and between them processed greater than 40,000 samples (~6% positivity rate). Here we summarise the laboratory set-up, design rationale, scope and processes deployed. This information can inform planning in response to future outbreaks.
Keywords
ebola; sierra leone; laboratories
Background
In December 2013, the first cases of EVD were being observed in Guéckédou, Guinea. Formal diagnosis of EVD was achieved in March 2014 through the European Mobile Laboratory (EMlab) response [1]. With the spread of EVD to Sierra Leone (SL) and Liberia during the summer of 2014, and an increasing death toll in the three affected countries, the ebola outbreak was declared a Public Health Emergency of International Concern (PHEIC) on the 8th August 2014 by the World Health Organisation (WHO) [2].
To assist the humanitarian response in Sierra Leone, the UK Ministry of Defence (MoD) were already in advanced planning stages to provide standard UK laboratory facilities in support of an Ebola Treatment Unit (ETU). This was designed to deliver a high western standard of health care to health workers in order to facilitate the international response. The UK response was enhanced further in September 2014, when Public Health England (PHE) was tasked by the UK Department for International Development (DfID) with providing laboratory-based EVD diagnostic support for three humanitarian Ebola Treatment Units (ETUs) that were being established in Sierra Leone. The health service there was under severe strain and the outbreak was spreading rapidly.
EVD usually presents with a non-specific fever, chills, and malaise, and can easily be confused with a range of other infectious diseases. Rapid and accurate diagnosis, with subsequent isolation and care of affected individuals, was seen as a critical step in controlling the ongoing epidemic. While the existing laboratory responses, such as the EMlab, were effective, they were designed for small-scale fast response outbreak scenarios [1]. Due to the severity of the outbreak, it became clear that larger-scale diagnostic facilities were urgently required to counter the growing epidemic. Combined laboratory response planning brought together MoD, PHE and Defence Science and Technology Laboratory (DSTL). Initial activity was focused in Kerry town, where the MoD and Save the Children facilities were being built, which was then extended to delivering larger diagnostic facilities at Makeni and Port Loko. These were linked to ETUs funded by the UK government.
Concept, Infrastructure, and Commissioning Timelines
Laboratories sited at geographically strategic locations served the populations of Freetown (Kerry Town), North East SL (Port Loko) and the Northern region of SL (Makeni), respectively. Based on estimates of the capacity required, the laboratories were scaled for an estimated throughput of up to 300 samples a day, a far greater capacity than existing arrangements, and as mitigation in event of worsening epidemic. It was envisaged that equipment provided in the laboratories would subsequently be made available to the government of Sierra Leone as part of a legacy, post-epidemic.
During the initial development period (from September 2014), intense activity was underway to establish laboratory facilities ahead of the opening of each ETU, in conjunction with the Non-Governmental Organisation (NGO) tasked with operating each ETU (Kerry Town: Save the Children [SAVE]; Port Loko: GOAL; Makeni: International Medical Corps [IMC]). The Kerry Town laboratory also supported the MoD ETU for healthcare workers, run on the same site, staffed by British and Canadian military medical personnel. This development period included: laboratory concept; production of Standard Operating Procedures (SOPs); Risk Assessments (RA) for both laboratory operation and wider staff-based RA; production of data-recording databases; procurement and logistics; training and procedure validation; and engagement with other personnel operating in country. These included: Sierra Leone government, WHO, MoD and other organisations.
Site infrastructure was supplied by either the British Military, and/or the relevant NGO at each site. For the laboratories, this infrastructure support included the provision of the buildings themselves, in addition to essential provision for chlorinated water supplies, electricity, catering, internet and communications, and operation of incinerators. Staff sleeping accommodation was sourced locally, or provided in tented facilities, separate from the ETUs. Within the diagnostic facilities, sanitary provision for staff was separated from the patient areas.
PHE opened EVD laboratories in Kerry Town (KT) on the 27th October 2014, with subsequent laboratories at Port Loko and Makeni opening on the 5th and 8th November 2014, respectively.
Laboratory build and design
While a tented facility was considered, a preferred option was a more robust concrete block and tin roof facility. This would provide a more controlled, contained, and secure working environment, and the capability for legacy and longer-term operation. The Kerry Town laboratory building was already under construction by the MoD in preparation for the UK MoD standard laboratory when PHE was asked to provide the laboratory capability; the building had to undergo some retrospective modifications to accommodate the testing workflow required. Learning from operating experiences at this site (including the need to remove sample reception bottlenecks and the increasing requirement to also support research studies) informed the development of the facilities at Makeni and Port Loko. These facilities, which were built by the MoD in conjunction with local contractors, were based upon a completely new, larger design specified by PHE.
The laboratory facilities were designed to permit processes involved in EVD diagnosis by real-time PCR, and malaria diagnosis using lateral flow devices. The final laboratory design that was settled upon in Makeni and Port Loko is illustrated (Figure 1). The laboratories were designed to be sited outside of the ETU highly restricted access zone, but adjacent to them to permit sample receipt from the ETU, or from community areas. They were sized approximately 12.75 m by 6.8 m, with designated areas for sample reception and sample processing, separate rooms for PCR mastermix set-up and template addition, while the main laboratory area housed nucleic acid extraction and equipment for clinical chemistry, haematology, coagulation and microbiology capability. In total, providing a capability to support the ongoing eradication programmes.
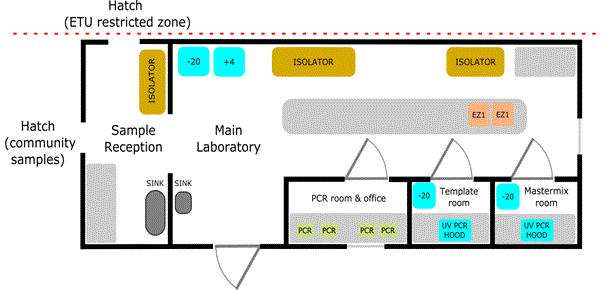
Figure 1. Schematic illustrating the design and typical layout of the EVD laboratories at Makeni and Port Loko. This differed from the earlier smaller laboratory at Kerry Town. There are separate rooms for handling PCR master mix, template (both equipped with UV PCR hoods) and performing PCR. The main laboratory space housed isolators for both primary sample processing and blood sciences capability, with additional laboratory workspace for platforms for PCR extraction and storage for samples and reagents. Hatches allowed receipt of samples direct from the ETU restricted-access zone or community samples from the lesser restricted area.
Air conditioning was stipulated as a requirement for both staff comfort and reliable operation of equipment, much of which had critical operating temperatures (e.g. the clinical chemistry platforms). Laboratories were supplied with two piped chlorinated water supplies at 125 ppm free chlorine for general hand washing and 5000 ppm chlorine for disinfection purposes. Experience gleaned on site indicated that, due to corrosion risk, the piped 5000 ppm chlorine supplies should be located external to the laboratory.
Biosafety
Ebola Virus (EBOV) is designated in the UK by the Advisory Committee on Dangerous Pathogens (ACDP) as a Hazard Group 4 (HG4) pathogen that must be handled under containment level (CL)-4 standards, (Biosafety Level, or BSL-4 in other countries). Sierra Leone is also an endemic Lassa Fever Virus (ACDP HG4) area, so it was conceivable that samples processed could contain either agent, in addition to other locally endemic pathogens. In Sierra Leone, it was impossible to rapidly create a BSL-4 laboratory infrastructure; therefore, it was essential that the laboratories were developed with safe working practises. Laboratories were designed to process samples in a similar and safe way to that employed in the UK. This included inactivation of the pathogenic organisms in the sample within primary containment, and subsequent removal of the inactivated sample to lower containment to allow high-throughput processing and analysis. Previous experience had shown that the bottleneck in this approach is the initial sample-inactivation stage.
Prior to the outbreak, many laboratories performing EVD diagnostics used a chaotropic salt-based (e.g. guanidine isothiocyanate) lysis buffer, such as Buffer AVL (Qiagen), or another manufacturer’s equivalent, to inactivate a sample. During planning stages, despite previous publications on the effectiveness of Buffer AVL on the inactivation of filoviruses, there were a number of unconfirmed reports, from several institutes operating in CL4 facilities, of the failure of Buffer AVL to fully inactivate EBOV. A subsequent paper from DSTL confirmed that Buffer AVL alone was not sufficient to completely inactivate EBOV in a representative clinical sample type [3]. With data from this study being available at the 2014 planning stage, a secondary processing step was therefore introduced after Buffer AVL treatment. Either a heat-treatment stage or ethanol stage (depending on the downstream RNA extraction method employed) provided an additional level of inactivation, and enhanced safety for the staff members who were likely to be processing large numbers of positive samples, with high concentrations of EBOV. It is noteworthy that, throughout the laboratories’ operation during the entire epidemic period, not a single laboratory acquired EBOV infection (i.e. known symptomatic or asymptomatic case) occurred in PHE-led laboratories. This was in a context with over 400 staff of mixed experience levels deployed in the laboratories, processing over 40,000 samples, including over 2400 positive samples, many of which were high-titre positive samples.
2021 Copyright OAT. All rights reserv
Methods and Equipment
Many of the initial mobile response teams employed manual processing for all stages of the testing process, which fits with the portable, responsive, nature of these capabilities. Under our enhanced scope, we purposely designed our workflows to use automation to free up staff time so that they could assist with other laboratory tasks. This also permitted increased consistency of results between staff members (particularly where different skill and experience levels were deployed), as well as increased sample throughput and reduced turnaround time.
Samples were received from the adjacent ETU, or from other local referral centres. The ideal sample type processed was whole EDTA blood from suspected EVD cases, which permitted direct use in the malaria lateral flow test and plasma extraction for RNA extraction and PCR. The initial plasma extraction and lateral flow test being carried out in flexible film isolators. Early on, many samples (especially those from the local referral centres) were received as clotted whole blood in plain tubes, which were difficult to separate and frequently severely haemolysed. Other sample-types analysed included swabs from cadavers, as well as semen samples and breast milk from recovered individuals during the latter stages of the outbreak. Early on in the response, wide variations in the quality and format of sample types received posed large problems in processing consistency, as did the integrity of samples, due to long delays in transporting samples to the laboratories. These problems were alleviated by the laboratories themselves, i.e. by supplying EDTA blood tubes to couriers to take back to the referring centres, until the local supply system established a reliable supply train and subsequent delivery of more-consistent samples.
Flexible film isolators were used for primary sample processing, providing a containment barrier. Isolators were sourced from PFI systems Ltd UK, and Solo containment Ltd, UK. The designs were metal-framed construction, with PVC envelope. The air system had battery backup and operated at negative pressure, with HEPA filtered inlet and double HEPA filtered outlet (Figure 2). The design accommodated gloved access and loading port to allow ingress and egress of materials (e.g. samples, waste etc) and housed power sockets for the equipment. Isolators were dedicated for primary sample processing with separate isolators housing other clinical chemistry equipment. Equipment used within the isolator, e.g. pipettes, racks, mini-centrifuge etc, was designated solely for that purpose. Isolator space was found to be the limiting factor in sample processing, so increased isolator provision was incorporated into the later laboratories. Isolators were wiped down internally with 5000 ppm hypochlorite, followed by diluted detergent, between uses; with 10,000 ppm hypochlorite solution reserved for spills. Heat blocks for further inactivation of samples post-Buffer AVL addition were housed outside, but adjacent to the isolator.
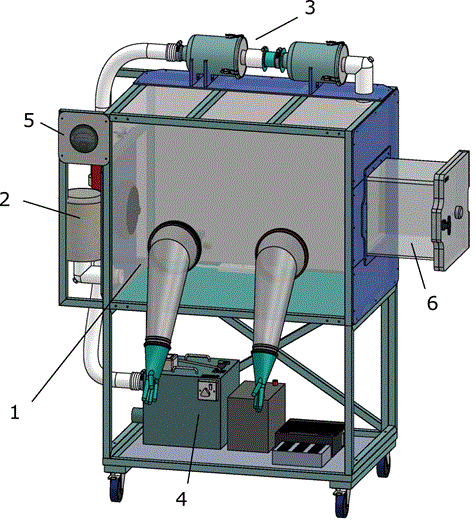
Figure 2. Schematic illustrating the flexible-film isolator used for sample processing. Comprising PVC envelope with gloved access (1); HEPA filtered Air inlet (2); Double HEPA filtered Air outlet (3); Air system with battery backup (4); Magnahelic manometer (Negative pressure gauge) (5) and Inlet and outlet port for addition and removal of samples/waste etc (6). Image supplied courtesy of PFI systems Ltd. UK. Main isolator canopy was approximately 1m wide x 0.72 m deep x 0.76 m high. For clarity, the gloves and sleeves are shown protruding from the isolator, when isolator is operational the gloves and sleeves protrude inwards.
Samples (contained in bags) were received by staff dressed in protective equipment (gown, apron, gloves and visor). After carefully disinfecting and checking the integrity of the outer packaging, samples were transferred to the isolator for further processing. Once samples were unpacked within the isolator, the sample details were checked against the sample tracking form and lab number assigned, prior to commencement of processing. Sample inactivation was started in the isolators by the addition of sample to Buffer AVL (Qiagen). Malaria testing, using lateral flow devices, was also carried out in the isolators, initially using the Binax Now malaria kit. The Sierra Leone Ministry of Health and Sanitation (MoHS) selected the SD malaria Antigen kit as its standard issue for its own facilities, and this was subsequently used by PHE for consistency with other laboratories operating in the region.
The first Kerry Town rotation initially extracted RNA from plasma samples using a manual method (QIAamp Viral RNA Kit, Qiagen). For these samples, ethanol was added to tubes (after addition of Buffer AVL) within the isolator to complete the inactivation procedure. In later rotations (all laboratories), and after commissioning of the deployed extraction platforms (Qiagen EZ1 Advanced XL), RNA extraction was automated. Inactivation was completed for these samples by heating Buffer AVL treated samples in heat blocks (60 oC; 15 min), located outside the isolators. Downstream extraction was completed from these samples using a Qiagen EZ1 virus 2.0 kit. The automated platforms were preferred for ease of training, consistency of results, reduced scope for error, reducing cross contamination, and increased throughput. Manual kits were retained in each laboratory for backup in event of machine failure, and for resilience. Separate rooms were designated for mastermix preparation, template addition and real-time PCR, with the mastermix preparation and template addition rooms equipped with UV-capable PCR hoods. Real-time PCR was performed on Cepheid Smartcycler or Roche LightCycler 96 platforms. The LightCycler 96 platforms were deployed to laboratories as a higher throughput maintenance-free real-time PCR platform. Real-time PCR predominantly used a PHE-variant of an existing Trombley Ebola Zaire nucleoprotein assay [4], targeting the NP region multiplexed with an MS2 bacteriophage internal control to control for extraction and inhibition [5].
Clinical chemistry and haematology instruments were deployed initially only to Kerry Town as part of the MoD laboratory, to support their facility and operated by military Biomedical Scientists (BMS) who were co-located at this laboratory. This equipment was later deployed to Makeni and Port Loko, as the clinical results obtained at Kerry Town proved they were of value to clinical management of EVD cases. Experience gained from the MoD operation in Kerry Town demonstrated how these additional capabilities were key to optimising patient management. They provided critical information to guide clinical decision-making, as well as longitudinal data for a condition for which, previously, there was little information [6,7].
The systems selected for deployment were the Fuji Dri-Chem NX-500 (blood biochemistry), the Horiba ABX Micro ES 60 (haematology) and the Hemochron Signature Elite (measuring coagulation). All three blood analysis systems were housed within a flexible-film isolator to ensure operator protection when processing potentially positive EBOV samples, with photographing of results and manual transcription of results onto the computer. It was noted that, for the clinical chemistry items to operate correctly, they required strict control of environmental temperature.
The majority of laboratory equipment & consumables was sourced in the UK (see logistics). Laboratory benching and furniture were built on-site using in-country craftsmen, with other ancillary items purchased locally.
PPE
Within the laboratories, a reduced Containment level 3 (CL3)-like level of operation was in process; samples were initially held in an isolator and inactivated before routine processing. The principle of the laboratory processing methodology and design were that operators would not come into direct contact with infectious material. Consequently, respiratory protective equipment and face visors were not required to protect against the infectious hazard. This had the additional benefit that staff could work for longer periods in a more comfortable environment, reducing the risks from fatigue and heat exhaustion, which could lead to errors. For routine operation and to provide a degree of separation from laboratory environments, the staff wore hospital scrubs and comfortable, lightweight footwear, beneath a laboratory gown. Staff also wore two pairs of gloves, and eye protection, which served to protect against chemical splashes (sodium hypochlorite). An enhanced level of protection was worn in the sample reception areas, which were typically located adjacent to/ or outside of the main laboratory and additionally consisted of disposable laboratory gown, splash-proof disposable apron, and face visor (supplementary material); FFP masks and goggles were also available.
Staffing
Staffing for laboratories was scaled at 12 team members per laboratory, to permit two shifts, typically 6 am – 2pm and 2 pm till 10pm with one team leader for each team, per shift. Cut off for reception of samples was 7 pm. Team levels were intended to permit a 1 day recuperation period per week, per team member, and to provide team resilience in event of staff sickness. Teams were deployed for a period of 4 weeks, with 1 week recuperation period back in the UK. Return to normal working duty was based upon further PHE criteria for returning workers, dependent on occupation. Team leads were identified from combination of experience, aptitude and performance, as deduced by trainer assessment during training, and were not necessarily the most-senior member of the team. Teams were designed to mix highly experienced skilled workers with less experienced workers, to enable the more experienced staff to ensure less experienced staff had reasonable oversight. During deployment, the team skill mix was critical, particularly in initial laboratory establishment. The presence of highly technically skilled and experienced staff was essential to assist in the establishment of the laboratories, and in troubleshooting platforms, assays and procedures that did not necessarily work optimally when deployed from the UK to more-austere locations.
Initially, laboratory staff were volunteers drawn from experienced staff in PHE, DSTL and the MoD, with subsequent deployments utilising staff requested from UK NHS laboratories, academic centres, and government laboratories following calls that were distributed through high level government. Staff usually had some laboratory experience, although not necessarily CL3 experience. Attitude and ability to cope and respond appropriately in an emergency were key requirements. Volunteers who came forward were asked to complete a selection form, requesting details of their scientific experience and foreign travel/work, together with a brief statement of their reasons for volunteering. Line managers were asked to comment on the volunteer’s abilities, trustworthiness, ability to cope in a crisis, and whether they had any physical or mental health issues. Volunteer selection was a two-step process: first based on the forms and, subsequently, by review of performance during training.
Training
Pre-deployment staff underwent an intensive 5-day training course, covering personal and laboratory safety, cultural awareness, laboratory processing techniques, and accident scenarios. An in-depth outline of the training regimen has been described [8] and was delivered in the UK in a purpose-built mock laboratory built to resemble the laboratories in Sierra Leone. On actual deployment, teams were planned to cross over with existing teams to ensure a period of handover, where practical. Laboratory workers were encouraged to feedback to the training team so that the training reflected operational realities. This also ensured that, like the response itself, the training was dynamic and evolved with the changing requirements. Training responded to newly adopted technologies, such as the blood sciences analysis equipment, or on other required aspects such as training. Dynamic changes included for example, training on appropriate packaging and storing of samples in-country to allow for subsequent research projects and evaluation of assays.
During operation, over 400 staff with a range of skill levels was trained and deployed. Security training was delivered to staff by the relevant NGO they were being deployed with.
Laboratory operation
Laboratories at the ETUs were in operation daily from opening to beyond the actual closure of the ETU in December 2015. Operating times were typically 6am to 10pm with peak maximal loads of approximately 200 samples a day. It was noted that the laboratories did not exceed the planned laboratory capacity of 300 samples daily, mainly as the process for transporting samples to the relevant laboratories was the practical bottleneck.
SOPs were developed during the planning stages prior to deployment and revised during operation, according to requirement, in consultation with UK-side support. Feedback from the laboratories was incorporated into the revisions. Within the laboratory, samples were tracked through the entire process using a sample tracking sheet, and unique labels assigned to each sample to ensure both that relevant procedures were being followed, and that samples were traceable.
Support & governance
A clinical lead and/or laboratory manager were deployed in-country to oversee the laboratories and co-ordinate between laboratories. Adverse laboratory incidents (e.g. sample processing errors, equipment failures) were logged in-country and reported to the in-country laboratory lead, who escalated back to the UK, where required.
A central email address was provided, and a telephone contact point, for support issues to be raised back to the UK depending on urgency. This provided a degree of continuity between teams, and a mechanism for more complex technical and or equipment issues to be resolved. Frequent telephone conference calls (typically bi-weekly to weekly) between UK support teams and SL were used to maintain awareness of the situation, to assess changing requirements and to co-ordinate research studies etc.
In-country equipment maintenance by manufacturers was not possible, since most had no service capacity in Sierra Leone. Therefore discussions and, where required, confidentiality disclosure agreements were held with various key manufacturers to provide PHE staff with access to basic servicing and troubleshooting capability for the various equipment items that had been deployed.
Information Technology
Access to on-site information technology capability was limited. Internet capability was supplied by the relevant NGO operating the ETU site. For routine sample tracking, laboratories were equipped with basic specification non-encrypted laptops. To minimise download times in remote locations, laptops were pre-loaded with relevant SOPS, operating manuals, equipment software backups, and bespoke database system. Laboratories were also equipped with a monotone multi-function printer/scanner/copier/fax to provide basic photocopying/fax and print capability.
A key requirement for control monitoring during the outbreak was that the laboratories were able to produce data outputs for distribution to the local and national co-ordinating centres. Existing off-the-shelf or emergency-use LIMS systems were briefly assessed but determined not to be viable options given the rapidly evolving situation and limited IT infrastructure. A bespoke database tracking system was developed using in-house PHE IT expertise. Critically, as networked IT services could not be guaranteed, this was developed in MS Access, and designed to operate in a stand-alone mode and to provide outputs that were compatible with the different reporting and data collation systems in use in the country. Data outputs were forwarded in-country using email to the respective co-ordinating centres. Data were also backed up remotely using a secure email to a UK-side data storage system.
Logistics
With their proven expertise in logistics and cold chain, the MoD Medical delivery team of M&GS initially sourced and procured all equipment on behalf of DfID. This was subsequently transferred to a commercial logistics company, Crown Agents, who utilised charter aircraft out of East Midlands airport, UK, to deliver to a central warehouse facility in Sierra Leone. In-country logistics were provided by IMC.
Biosecurity
The laboratories were kept under 24-hour guard using locally employed security staff to protect the equipment, staff and samples. Laboratories were locked when not in use.
Research and Ethics
Although the PHE-operated laboratories were established to provide frontline diagnostics to help bring the EVD outbreak under control, frequent research requests were received by the laboratories, and came through a series of routes, from informal requests direct to laboratory staff to formal approaches to PHE, NGOs, military, DfID etc. The broader research community was also demanding access to data and laboratories either for access to samples or to directly support clinical trials or trial diagnostic tests [9]. Given the range of demands that were being placed on already-stretched laboratories and staff supporting the response, a mechanism was set up to prioritise the use of the facilities, with the proviso that any research activities were secondary to the diagnostic turnaround. Research studies were co-ordinated through and agreed with the laboratory team lead, in-country clinical lead, PHE in the UK, and DfID; supported studies had to demonstrate ethical clearance, both institutional and from the Sierra Leone Ethics and Scientific Review Committee. PHE laboratories successfully provided direct support for a range of studies, including those published [5,10-13] and, with MoHS approval, provided samples to others (including the FIND organisation) to permit evaluation of novel EVD diagnostics.
Decommissioning & Legacy
Once the EVD epidemic came under control, the ETUs were decommissioned. At each ETU, the laboratories were some of the last facilities to close, ensuring that community-derived samples could continue to be processed. The laboratories were then decommissioned at the ebola treatment unit locations. The laboratories were moved to new locations as part of a legacy plan to provide diagnostic capability to the Sierra Leone health system. This process was audited, with full logging of materials and equipment to ensure all items were made safe, and either destroyed by incineration or decontaminated with hypochlorite.
Conclusions
The operation of diagnostic laboratories was a key milestone in controlling the West African ebola outbreak in 2014-2015. In Sierra Leone, three PHE-led laboratories operating in Makeni, Port Loko and Kerry Town. They played a significant role in this response, by providing rapid primary diagnostic facilities to identify ebola cases, thus allowing their isolation and treatment. The laboratories were also able to support some research studies that contributed evidence in support of novel EVD diagnostics and treatments.
Mounting a response of this magnitude was a massive logistical operation set against a highly dynamic situation. In particular, the early planning stages required the combined input and co-operation of the MoD, DSTL and PHE to rapidly establish the laboratory facilities, staff and procedures and provide the immediate capability that was urgently required. The response to challenging timelines would not have been possible without that combined effort. The subsequent maintenance of the response further called on multiple sectors, and required the co-ordination of many different teams, both from support workers located in the UK, to those deployed in-country themselves. This work provides a model for managing future outbreaks of disease. No single organisation was able to deal with an emergency on this scale in isolation. The bringing together of PHE, DSTL and MoD enabled the UK response to draw on the strengths of these organisations, which synergised effectively to deliver the capability outlined in this paper.
Throughout this operation, key difficulties arose, and are outlined further in the ‘lessons learnt’ article in “Mobile laboratory experience in the Ebola outbreak in Africa, diverse concepts and common issues”. Well-organised logistics were paramount to the resolution of some these issues. Mechanisms to sustain the response and ensure continuity between rotating staff members were also critical, as was maintaining communication channels in a highly dynamic situation.
This work has demonstrated how laboratories can be established and function safely to contribute to the control of an epidemic of a highly pathogenic organism in a difficult environment and under extreme pressures.
Whilst the outbreak has now been controlled, with Sierra Leone finally declared ebola free on 17th March 2016, the onus is now on us to ensure that we are able to use the experience gained, and the lessons learnt, to provide a lasting legacy to prevent the tragic situation arising again.
Acknowledgements
The EVD laboratory operation was funded through the Department for International Development.
Operation of the laboratories and ETUs in SL was an immense operation involving hundreds of individuals drawn from across many areas of society, to deal with a tragic epidemic. We offer our deepest gratitude to the many individuals who selflessly contributed their time and resources both on the ground and behind the scenes, including those who stepped in to fill the roles of colleagues contributing to the response. We thank all those involved both in the UK and overseas for their magnificent contribution to this major humanitarian effort.
References
- Wolfel R, Stoecker K, Fleischmann E, Gramsamer B, Wagner M, et al. (2015) Mobile diagnostics in outbreak response, not only for Ebola: a blueprint for a modular and robust field laboratory. Euro Surveill, 20 (44): 1-9.
- World Health Organisation (2014) Statement on the 1st meeting of the IHR Emergency Committee on the 2014 Ebola outbreak in West Africa. In: Committee, I. E. (ed.). World Health Organisation.
- Smither SJ, Weller SA, Phelps A, Eastaugh L, Ngugi S, et al. (2015) Buffer AVL alone does not inactivate Ebola virus in a representative clinical sample type. J. Clin Microbiol 53: 3148-3154.
- Trombley AR, Wachter L, Garrison J, Buckley-Beason VA, Jahrling J, et al. (2010) Comprehensive panel of real-time TaqMan polymerase chain reaction assays for detection and absolute quantification of filoviruses, arenaviruses, and New World hantaviruses. Am J Trop Med Hyg 82: 954-960.
- Weller SA, Bailey D, Matthews S, Lumley S, Sweed A, et al. (2016) Evaluation of the Biofire FilmArray BioThreat-E Test (v2.5) for Rapid Identification of Ebola Virus Disease in Heat-Treated Blood Samples Obtained in Sierra Leone and the United Kingdom. J Clin Microbiol 54: 114-119.
- Hunt L, Gupta-Wright A, Simms V, Tamba F, Knott V, et al. (2015) Clinical presentation, biochemical, and haematological parameters and their association with outcome in patients with Ebola virus disease: an observational cohort study. Lancet Infect Dis 15: 1292-1299.
- O'Shea MK, Clay KA, Craig DG, Matthews SW, Kao RL, et al. (2015) Diagnosis of Febrile Illnesses Other Than Ebola Virus Disease at an Ebola Treatment Unit in Sierra Leone. Clin Infect Dis 61: 795-798. [crossref]
- Logue CH, Hawkey S, Lansley A, Fraser S, Shieber C, et al. (2017) Design and Implementation of Ebola Diagnostic Training for Scientists Deploying to PHE Field Laboratories in Sierra Leone - Oct 2014 – Aug 2016. Philosphical Transactions of the Royal Society B 372.
- Lang T (2015) Ebola: Embed research in outbreak response. Nature 524: 29-31. [crossref]
- Semper AE, Broadhurst MJ, Richards J, Foster GM, Simpson AJ, et al. (2016) Performance of the GeneXpert Ebola Assay for Diagnosis of Ebola Virus Disease in Sierra Leone: A Field Evaluation Study. PLoS Med 13: e1001980.
- Broadhurst MJ, Kelly JD, Miller A, Semper A, Bailey D, et al. (2015) ReEBOV Antigen Rapid Test kit for point-of-care and laboratory-based testing for Ebola virus disease: a field validation study. Lancet 386: 867-874.
- Walker NF, Brown CS, Youkee D, Baker P, Williams N, et al. (2015) Evaluation of a point-of-care blood test for identification of Ebola virus disease at Ebola holding units, Western Area, Sierra Leone, January to February 2015. Eurrro Surveill. 20(12). pii 21073.
- Mattiuzzo G, Ashall J, Doris KS, Maclellan-Gibson K, Nicolson C, et al. (2015) Development of Lentivirus-Based Reference Materials for Ebola Virus Nucleic Acid Amplification Technology-Based Assays. PLoS One, 10, e0142751.


