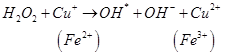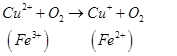Abstract
Berries are often tagged ‘super fruits’ and command premium prices due to their purported high concentration of antioxidants and vitamin C. This study sets out to support or dispute the above assertion through a comparative analysis of the total phenolic and vitamin C in cultivated fresh (variety; Draper, Duke and Brigatta) and freeze dried blueberry coupled with the determination of the antioxidant capacity of their extracts. DCPIP, Folin-Ciocalteu and DPPH assays were used. The total phenolic content ranged from 262.64 to 287.96 mgGAE/100 gms in the fresh berries and was 426.06 mgGAE/100 gms in the freeze dried samples. The amount phenolic content of the freeze dried blueberries is significantly higher than in the fresh blueberries. Thus the apparent antioxidant health benefit of the freeze dried berries outweighs that of the fresh berries. The fresh samples had an average measurement of 10.9 mg/100 gms of vitamin C with a small variance and the total antioxidant activity ranged from 28% to 49%.
Key words
blueberries, antioxidants, total phenolic, antioxidant activity, vitamin C, DPPH, DCPIP and Folin-Ciocalteu assay.
Introduction
Blueberries belong to the berry family Vaccinium corymbosium. They are divided into three major groups that include wild berries – low bush, cultivated and rabbiteye – highbush. North America is the world leader in blueberry production with other continents following with the trend including Europe, China and Africa [1]. Studies on the content and physiological activity of the compounds and nutrients in blueberries have quantitatively been documented over the years showing the benefits they possess [2]. Blueberries contain bioactive compounds such as anthocyanin, phenolic acid, tannin, carotenoids, vitamin A, C, and E, folic acid and minerals [3]. Of the metabolites found in blueberries, the polyphenols have been researched and reviewed the most thus showing the value given to the bioactive compounds in blueberries and their overall benefits. Other contributors to the antioxidant capacity of blueberries include flavonoids (quercitin and its derivatives) and phenolic acids (caffeic acid, chlorogenic acid, p-coumaric and ferulic acid), all of which help in the prevention of oxidative stress and act as anti-aging compounds [2]. The structure, properties and functions of these compounds as antioxidants, have been reviewed extensively [4-6]. As antioxidants, they protect the cardio-vascular system from damage caused by free radicals in the human body and extend the shelf life of fat-containing foods through retardation of oxidative [7]. The roles of vitamin C are manifested through its functionality as a reducing agent and co-enzyme in several metabolic pathways [8]. Similarly, polyphenols (phenolics) are directly related to the sensory characteristics in most fruits and vegetables such as flavour, astringency and colour. The presence of this compounds act as antioxidants, anti-inflammatory, anti-allergic and prebiotic properties. They inhibit micro-organisms in the human body [9]. Antioxidant activity in blueberries is linked to the presence of phenolic compounds which have hydrogen donating and the metal ion chelating abilities. These compounds have been studied in vitro and have been found to have potential health benefits brought about by their antioxidant activity [10].
Investigations among blueberries growers indicate a focus on quality and nutritional content as ways of enhancing bioavailability through investment in breeding technology [11]. This is brought about by the quality of different varieties, total phenolic content and antioxidant activity [12]. Vitamin C is also an important component in blueberries. These micro-nutrients are abundant in blueberries and act as neutralisers of free radicals in the human body. The importance of the phytochemicals and vitamin C present has propelled the blueberry to a ‘super fruit’ status globally. Recent epidemiological studies have shown that many dietary phenolic compounds derived from blueberries are very effective antioxidants in-vitro which significantly contributes to their protective effects in-vivo. A high amount of antioxidants is found in the pigmentation of most fruits and vegetables which is commercially extracted to act as preservatives.The objectives of this study are to determine the antioxidant capacity, total phenolic and ascorbic acid content of fresh and freeze dried blueberries.
Ascorbic acid
Ascorbic acid also known as vitamin C (C6H8O6) is a potent water soluble antioxidant and is naturally found in high amounts in fruits and vegetables. It is a six carbon compound related to glucose. It is an essential nutrient in the human diet as it helps in the absorption of iron, calcium and folic acid and maintenance of the connective tissues and bones, hence prevention of scurvy and a major component of cold and flu remedies. L-ascorbic is commonly used in its synthetic form in the food industry as a supplement and preservative. Primarily the human body cannot synthesise vitamin C hence the main source is through eating plenty of fruits and vegetables regularly. The roles of vitamin C are manifested through its functionality as a reducing agent and co-enzyme in several metabolic pathways [8].
Polyphenols are phytochemicals that have a common structure, a flavone backbone. Three main categories are recognised; flavonoids, phenolic acids and stilbenes. Most polyphenols belong to the flavonoid subclass that contains two or more aromatic rings each bearing one or more phenolic hydroxyl groups and connected by a carbon bridge.
They are further divided into sub-classes including flavonoles, isoflavanoles, anthocyanins, catechins and others. The phenolic acids are divided into two which include those derived from benzoic acid (comprising seven carbon atoms) and from cinnamic acid (comprising of nine carbon atoms). Polyphenols are directly related to the sensory characteristics in most fruits and vegetables such as flavour, astringency and colour. The presence of this compounds act as antioxidants, anti-inflammatory, anti-allergic and prebiotic properties. They inhibit micro-organisms in the human body [9].
Natural are phytochemicals, vitamins and other nutrients that protect the cardio-vascular system from damage caused by free radicals in the human body and extend the shelf life of fat-containing foods through retardation of oxidative rancidity. Other forms of antioxidants include melatonin, metal binding proteins, reduced co-enzyme Q, catalase, superoxide dismutase, glutathione peroxidase and peroxiredoxins [7], and synthetic phenol derivatives such as butylated hydroxyanisole and butylated hydroxytoluene.
Antioxidants retard oxidative processes through several mechanisms; hydrogen donation, singlet oxygen quenching, enzyme inhibition, UV absorption peroxide decomposition and metal chelation [7]. Primary antioxidants naturally donate an electron to free radicals thereby converting them into harmless molecules thereby slowing down and preventing oxidation. Reduced co-enzyme Q acts by trapping both the superoxide radical (O2) and the perferryl radical Fe3+-O2 [13] as shown below
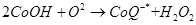 (1)
(1)
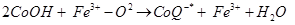 (2)
(2)
Antioxidant activity in blueberries is linked to the presence of phenolic compounds which have hydrogen donating and the metal ion chelating abilities. These compounds have been studied in vitro and have been found to have potential health benefits brought about by their antioxidant activity [10]. The antioxidant catalysis of hydroxyl radical formation from superoxide and hydrogen peroxide by iron and copper ions [14] is shown below.
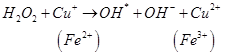
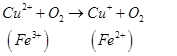
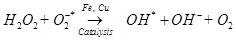
Various methods have been developed to determine and estimate the concentrations of antioxidants and vitamin C in berries and other fruits and vegetables [15,16]. The preparation and extraction of samples is done using various solvents such as distilled water, ethanol, methanol and acetone at different concentrations [17-19]. In order to reach optimization during the extraction process, distilled water is used to increase the efficiency of the solvent. Moreover the extraction of antioxidants requires polar solvents, which are able to ionize the solution, this include solvents such as methanol or acids [20]. There are various methods that are used in determining the total antioxidant activity and vitamin C, this include FRAP (Ferric Reducing Antioxidant Power), TEAC (Trolox Equivalent Antioxidant Capacity), ORAC (Oxygen Radical Absorbance Capacity), DPPH (2,2 Diphenyl-picrylhydrazl), DCPIP, and Folin-Ciocalteu [15,16,21].
DCPIP method
This is a volumetric analysis [22] that exploited the reducing property of ascorbic acid (vitamin C). In the presence of the redox dye DCPIP (2, 6-dichlorophenol-indophenol), ascorbic acid is oxidised as shown (Figure 1) and the blue DCPIP is reduced to a colourless compound. This is the most effective way of measuring vitamin C compounds in blueberries and is easily replicable [22]. The method is cheap, simple, quick and reliable for estimation of ascorbic acid in fruit juices, but may be less accurate and more so with very coloured fruit and vegetable juices.
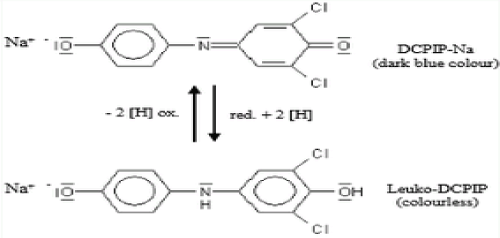
Figure 1. Ascorbic acid reduction of DCPIP
Folin-Ciocalteu assay
This method is often used to determine the total phenolic in fruits, using gallic acid as a standard. Folin-Ciocalteu reagent reduces the capacity by oxidation of the proteins and polyphenols [23,24] through a redox reaction, the reagent is reduced to a blue colour under alkaline conditions [24]. The concentration of the phenolic is then determined by measuring the absorbance spectrophotometerically at 740 nm and applying the Beer-Lambert law (Abs = ԑbc) which states that, the absorbance (Abs) is equal to extinction coefficient (ԑ) times the pathlength (b) and the concentration of the solution of interest (c).
DPPH (2, 2- Diphenyl-picrylhydrazl) assay
The DPPH assay tests the ability of compounds to act as free radical scavengers or hydrogen donor, thus it has been used in many studies to monitor the scavenging activities of antioxidants in lipophilic systems [25] and the antioxidant activity of the phytochemicals [26]. The method is based on the principle of electron transfer which produces a violet solution in methanol and gives a strong absorption maximum at 515 nm. A colour change is observed as the molar absorption of DPPH reduces when the odd electron pairs with hydrogen from the antioxidants form the reduced DPPH-H (Figure 2).
Thus the colour intensity is proportional to the degree of inhibition.
The concentration of the sample can be determined by using a standard curve and / or the Beer-Lambert law (Abs=ԑbc). It is accurate, sensitive and economical in evaluating antioxidant activity.
Materials and methods
Materials
Analytical grade reagents were purchased from Fisher Scientific Loughborough UK (DCPIP standard, 20% m/v metaphosphoric acid, ethanol and L-ascorbic acid, copper sulphate (CuSO4), potassium sodium tartrate (KNaC4H4KNaO6), sodium hydroxide (NaOH), ethanol (C2H6O) and Gallic acid (C7H6O5) and Sigma Aldrich Company Ltd, Dorset, UK (Folin-Ciocalteu reagent, methanol and 2, 2-diphenylpicrylhydrazil (DPPH).
The blueberries (Table 1) were purchased form a local supermarket in London, UK.
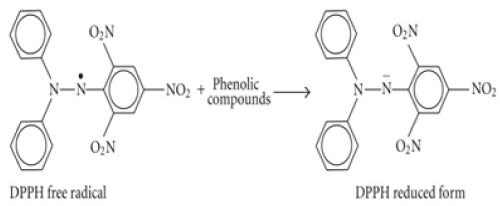
Figure 2. DPPH inhibition by antioxidant odd electron pairs with hydrogen from the antioxidants
Table 1. List of samples used
Sample |
Variety |
Country of origin |
1 |
Duke |
Chile |
2 |
Brigatta |
Chile |
3 |
Draper (organic) |
Chile |
4 |
Freeze dried |
California |
Sample preparation
For the experimentation (DPPH, DCPIP and Folin-Ciocalteumethods), 5 grams of blueberries in three replicates each was extracted by homogenizing the sample with an ultra turrax micra D-9 digitronic blender with acidified methanol containing 85:15 v/v, (MeOH:HCl) according to a method advanced by El-Sayed and Huel [27]. The homogenate was centrifuged at 3500 RPM for ten minutes and the separation was made. The supernatant was of red colour and was diluted with distilled water by a factor of 10 to make a 100 ml solution. All samples were stored in a refrigerator at 4oC.
Analytical procedures
Determination of ascorbic acid by DCPIP: A 0.05% solution of DCPIP was prepared with distilled water and similarly, a 0.2 mg cm-3 solution was prepared by dissolving 0.05 g of L-ascorbic acid in 60 ml of 20% metaphosphoric acid and diluted with 250 ml distilled water. The DCPIP solution was standardised with a known concentration of ascorbic acid, followed by titration of 10 ml of blueberries solutions against the DCPIP solution. All analysis were in triplicate. The ascorbic contentment was calculated as follows;
Mass =MR (Ascorbic acid)×C (DCPIP)×V (DCPIP) (6)
where MR=Molar Mass, C=Concentration and V=Volume
Determination of total phenolic by the Folin –Ciocalteu assay: The Folin- Ciocalteu reagent was prepared thus; 5 ml of Lowry reagent composed of (1 ml CuSO4 as a 1% solution, 1 ml of potassium titrate as a 2% solution and 100 ml of the mixture containing 20 mg/ml of sodium carbonate and 4 mg/ml of sodium hydroxide) was added to 1 ml of the blueberry samples. After 5 minutes 1 ml of Folin-Ciocalteu reagent was added and stirred using a vortex SA6 stirrer. The mixture was then incubated for 2 hours and the absorbance of the resulting colour was measured at a wavelength of 740 nm using a spectrophotometer.
Quantification was carried out on the basis of the standard curve of gallic acid and results are expressed as GAE mg/ml (Gallic Acid Equivalents). Measurements were done in triplicate. Gallic acid was used as standard and was prepared by making a solution of 0.01 g of Gallic diluted with 10 ml of methanol to make a 1 mg/1 ml solution. Absorbance was recorded and results were as below (Table 2).
The resulting absorbance was plotted against their concentration (Table 2). An external calibration was used for calculation of total phenol content of the blueberries samples. The equation against the standard curve is y = 3.87x – 0.1444 *(where y is absorbance and x is concentration) with the R2 set at 0.997.
Antioxidant activity by DPPH assay: The DPPH scavenging activity assay was done according to a method reported by [26]. A solution was freshly prepared from 80 mM (0.032mg) of DPPH in 95% methanol to make 100ml solution. This was stored in the dark. A control was also prepared using 3.5ml methanol and 0.3ml DPPH solution a reading was taken. The sample was the prepared by mixing 0.5ml of the blueberry sample with 3ml of methanol and addition of 0.3ml of DPPH solution in replicate. Absorbance was measured in triplicate at 515nm after 30 minutes. The antioxidant activity was calculated as follows
% DPPH scavenging activity = (1 – [Asample /Acontrol t=0])×100) (7)
*where A = Absorbance and t = time
Table 2. Standardization of gallic acid
Conc. of standard Gallic acid(mg/ml) |
Average of absorbance λmax=740 nm |
0.10 |
0.237 |
0.15 |
0.455 |
0.20 |
0.606 |
0.25 |
0.836 |
0.30 |
1.014 |
Statistical analyses
The data are expressed as mean ± standard deviation (SD) from three parallel measurements. Significance of difference was defined at the 5% level (P˂0.05).
Results and discussion
1ml of the blueberry sample was reduced by a factor of 25 with distilled water and titrated by 0.05mg/ml DCPIP reagent to get a titre of 0.3ml and 0.4ml (Table 3) [16,28-30].
Among the varieties analysed for ascorbic acid the ‘Brigatta’ showed a high ascorbic acid level at 13.1 mg/100gms as compared to the other two samples, ‘Draper’ and ‘Duke’ which had vitamin C levels at 9.8 mg/100gms. The results were inconclusive due to the fact that the natural pigmentation of the anthocyanin was dark red after extraction hence it was difficult to get a very clear sample. In order to achieve a clear sample, sodium or potassium silicate would have been used as a discoloration compound. Most experimentation carried out have shown blueberries to contain vitamin C capacity ranging from 3mg to 18mg per 100grams [16,28]. Vitamin C is an essential antioxidant that helps in neutralising free radicals. The average recommended daily allowance (RDA) for adults is 40mg/day. Antioxidant effects of vitamin C present in blueberries has been demonstrated in vitro along with vitamin E, K, beta carotene, manganese and potassium to act as reducing agents.
The spectrophotometer was calibrated using the Folin-Ciocalteu reagent and a reading of zero (0) was recorded. Then the total phenolic (TP) present in the blueberries was calculated using the average absorbance after two hours using the equation (y = 3.87x- 0.1444) *where y = absorbance and x = concentration. Hence the results were calculated as mean ± standard deviation (Table 3).
Phenolic compounds make the highest amount of compounds in fruits and vegetables. They are also known to have antibacterial effects, anti-inflammatory activities and detoxification ability [31]. The TPC of the varieties analysed showed higher levels in the freeze dried ‘California’ variety at 427.06mgGAE/100g, ‘Draper’ variety at 287.96 mgGAE/100g followed by the ‘Duke’ variety at 262.98mgGAE/100g and the Brigatta variety at 241.41mgGAE/100g. When all the varieties were compared there was a significant difference (p˂ 0.05) observed in their TP levels especially between the fresh and the freeze dried variety. The highest levels of TP were consistently found in extractions of freeze dries blueberries [32].
The TPC levels obtained from the experiment are comparable to previous findings which have reported average values between 181 – 310 mgGAE/100g on cultivated varieties of blueberries [28,33]. Results observed on wild blueberries showed a higher TPC with levels ranging between 577-1054 mgGAE/100g [29]. The fresh variety samples were stored in the dark for 24 hours and inhibition was recorded at 740nm showing a rise in TPC levels ranging between 419.60 – 490.45 mgGAE/100g. The organic ‘Draper’ variety recorded a high amount of TPC at 490.45 mgGAE/100g. These results showed a significant difference in the four varieties of blueberries.
The DPPH method was used to evaluate the scavenging properties (scavenging activity) of the antioxidants present in blueberries and results are as shown (Table 3). The samples showed very strong scavenging activity against DPPH after 30minutes with the organic freeze dried blueberry displaying 49%, while the organic ‘Draper’ and ‘Duke’ variety ranged between 43% and 28% respectively. The data collected are in agreement with previous studies which showed results in different varieties of blueberries ranging between 28% - 60%[30]. This proved that blueberries are a good source of antioxidants which are used in eliminating and protecting cells against oxidative stress and fighting free radicals. The organic freeze dried blueberry displayed a higher level of antioxidant activity (p˂ 0.05) as compared to the other varieties. Sustainably grown and organic freeze dried blueberries contained higher levels (49%) as compared to the organic fresh and conventionally grown blueberry (43% and 28%). Furthermore the results showed that organically grown fruits and vegetables contain high antioxidant activity than cultivators however this has not been studied conclusively [34].
Table 2. Standardization of gallic acid
Conc. of standard Gallic acid(mg/ml) |
Average of absorbance λmax=740 nm |
0.10 |
0.237 |
0.15 |
0.455 |
0.20 |
0.606 |
0.25 |
0.836 |
0.30 |
1.014 |
Conclusion
The results demonstrate a statistically relevant trend of higher levels of total phenolic and antioxidant activity in organically grown blueberries especially the freeze dried variety. The outcomes from the various methods used indicate that blueberries have some of the highest total antioxidant content in comparison to other fruits in the market. Freeze drying as a method of increasing the shelf life in fruit processing is based on dehydration by sublimation. Due to the absence of water and the low temperatures used, the product has a long shelf-life and in addition the product maintains its antioxidant metabolites and the sensory quality [34]. The antioxidant activity in blueberries is closely related to the amount of phenolic compounds present.
Awareness of the health benefits and antioxidant activity has impelled the sale and consumption of the blueberry. The total phenolic content is the highest contributor of antioxidants in blueberries as compared to the vitamin C content. The methods used are in vitro assays which give cohesive parameters rather than individual antioxidant calculations; hence it is very hard to determine the amount of antioxidant needed to reduce excessive reactive oxygen species (ROS). The amount of vitamin C indicated on blueberry packets (16mg/100g) vary in most varieties in the supermarkets due to various factors that include soil type, temperature and agronomic conditions.
Acknowledgement
This study was carried out at the School of Applied Sciences laboratories, London South Bank University, UK.
References
- Srivastava A, Akoh CC, Fisher J, Krewer G (2007) Effects of anthocyanin fractions from selected cultivators of Georgia grown blueberries on apoptosis and phase II enzymes. J Agric Food Chem 55: 3180-3185. [Crossref]
- Taruscio TG, Barney DL, Exon J (2004) Content and profile of flavonoid and phenolic acid compounds in conjunction with the antioxidant capacity for a variety of north west vaccinium berries. J Agric Food Chem 52: 3169-3176. [Crossref]
- Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, et al. (2001) Chemoprevention of oesophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res 6: 6112- 6119. [Crossref]
- Grzesik M, Naparlo K, Bartosz G, Sadowska-Bartosz I (2018) Antioxidant properties of catechins: Comparison with other antioxidants. Food Chemistry 241: 480-492.
- Liu J, Liu S, Chen Y, Zhang L, Jin C (2017) Physical, mechanical and antioxidant properties of chitosan films grafted with different hydroxybenzoic acids. Food Hydrocolloids 71: 176-186.
- Lu X, Li N, Qiao X, Qiu Z, Liu P (2017) Composition analysis and antioxidant properties of black garlic extract. J Food Drug Anal 25: 340-349.
- Pisoschi AN, Pop A (201) The role of antioxidants in the chemistry of oxidative stress: A review. Eur J Med Chem 97:55-74. [Crossref]
- Smirnoff N (1996) The function and metabolism of ascorbic acid in plants. Annal Bot 78: 661-669.
- Rice-Evans CA, Miller NJ, Paganga G (1995) Structure–antioxidant activity relationships of flavanols and phenolic acids. Free Radical Biology and Medicine 20: 933-956.
- Alam N, Bristi NJ, 2021 Copyright OAT. All rights reserv and in-vitro methods evaluation of antioxidant activity. Saudi Pharmaceutical Journal 21: 143-152.
- Scalzo J, Politi A, Pellegrini N, Mezzetti B, Battino M (2005) Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 21: 207–213.
- Kalt W, Ryan DAJ, Duy JC, Prior RL, Ehlenfeldt MK, et al. (2001) Interspecific variation in anthocyanins, phenolics and antioxidant capacity among genotypes of highbush and low bush blueberries (vaccinium section cyamicoccus spp.). J Agric Food Chem 49: 4761-4767. [Crossref]
- Beyer RE (1992) An analysis of the role of co-enzyme Q in free radical generation as an antioxidant. BioChem Cell Biology 70: 390-403. [Crossref]
- Halliwell B, Aeschbach R, Lolinger J, Aruoma IO (1995) The characterization of antioxidants. Food Chem Toxicol 33: 601-607.
- Prior LR, Wu X, Schaich K (2005) Standardised methods for the determination of antioxidant capacity and phenolics in food and dietary supplements. J Agric Food Chem 53: 4290-4302. [Crossref]
- Wang H, Cao G, Prior RL (1996) Total antioxidant capacity of fruits. J Agr Food Chem 44: 701-705.
- Laguerre M, Lacomte J, Villeneueve P (2007) Evaluation of the ability of antioxidants to counteract lipid oxidation. Existing methods, new trends and challenges. Prog Lipid Res 46: 244-282. [Crossref]
- Sanchez-Moreno C, Larrauri J, Saura-Calixto F (1991) Free radical scavenging capacity and inhibition of lipid oxidation of wines, grape juices and related polyphenolic constituents. Food Res Int 32: 407-412.
- Antolovich M, Prenzler PD, Patsalides E, McDonald S, Robards K (2002) Methods for testing antioxidant activity. Analyst 127: 183-198. [Crossref]
- Goristein S, Caspi A, Zemser M, Trakhtenberg S (2000) Comparative contents of some phenolics in beer, red and white wines. Nutr Res 20: 131-135.
- Benzie IF, Strain JJ (1996) The Ferric Reducing Ability of Plasma (FRAP) as a measure of ‘Antioxidant Power’: The FRAP Assay. Anal Biochem 239: 70-76. [Crossref]
- Kabasakalis V, Siopidou D, Moshatou E (2000) Ascorbic acid content of commercial fruit juices and its rate of loss upon storage. Food Chem 70: 325-328.
- Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Enzymol 299: 152-178.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdicphosphotungstic acid reagents. Am J Enol Vitic 16: 144-158.
- Singh S, Singh RP (2008) In-vitro methods of assay of antioxidants: An overview. Food Rev Int 24: 392-415.
- Brand-Williams W, Cuvelier ME, Berset C (1995) Use of free radical method to evaluate antioxidant activity. Lebensm Wiss Technology 28: 25-30.
- Abdel-Aal el-SM, Hucl P (2003) Composition and stability of anthocyanins in blue-grained wheat. J Agric Food Chem 51: 2174-2180. [Crossref]
- Prior RL (1998) Antioxidant capacity and health benefits of fruits and vegetables: blueberries, the leader of the pack. In Proceedings of the 32nd Annual Open House North Carolina Blueberry Council (pp. 3-12). Portland: US Highbush Blueberry Council.
- Giovanelli G, Buratti S (2009) Comparison of polyphenolic composition and antioxidant activity of wild Italian blueberries and some cultivated varieties. Food Chem 112: 903-908.
- Li W, Hydanmaka AW, Lowry L, Beta T (2009) Comparison of antioxidant capacity and phenolic compounds of berries, chokecherry and seabuckthorn. Cent Eur J Biol 4: 499–506.
- Tuohy KM, Conterno L, Gesperotti M, Viola R (2012) Up-regulating the human intestinal microbiome using whole plant foods, polyphenols and/or fibre. J Agric Food Chem 60: 8776-8782. [Crossref]
- Woese K, Lange D, Boess C, Bogl KWA (1997) A comparison of organically and conventionally grown foods: Results of a review of the relevant literature. J Sci Food Agri 74: 281-293.
- Dragovic-Uzelac V, Savic Z, Brala A, Levaj B, Bursac-Kovacevic D, et al. (2010) Evaluation of phenolic content and antioxidant capacity of blueberry cultivators (Vaccinium corymbosum L.) grown in Northwest Croatia. Food Technol Biotech 48: 214-221.
- Asami DK, Hong YJ, Barret DM, Mitchell AE (2003) Comparison of the total phenolic and ascorbic acid content of freeze dried and air dried Marion berry, strawberry and corn grown using conventional, organic, and sustainable agricultural practices. J Agri Food Chem.