Abstract
Background and Aim: Klotho, as anti-ageing hormone, is involved in vascular health through regulating endothelial cell function and oxidative stress. We designed a cross-sectional study to test the relationship between Klotho and CAD in Chinese adults.
Methods and results: A total of 100 patients who underwent coronary angiography were enrolled and divided into two groups. One group consisted of patients with CAD (n=50) and the other was without CAD (n=50). Serum Klotho was acquired by using ELISA. Serum Klotho levels were significantly descended in the CAD group, as compared with the non-CAD group (443.0±29.8pg/ml vs.616.5 ±29.4 pg/ml, P<0.001). Multiple logistic regression analysis revealed that a 100 pg/ml reduction of Klotho level was associated with mean 30% increase in CAD incidence.
Conclusions: Serum Klotho levels was significantly lower in patients with CAD. We conclude that deficiency of Klotho in patients may be an important predisposing factor for CAD.
Introduction
Despite significant advances in respect of prevention and treatment, Coronary artery disease (CAD) remains the principal cause of mortality worldwide [1]. Unhealth life style, such as lack of exercise, smoking, and a diet of energy-dense fast food, have be quantified as classical risk factors of cardiovascular disease (CVD), but the susceptibility and progression of CAD is not completely elucidated. Recently, many new biological systems raised to provide additional information about CVD which improve our understanding of atherosclerotic pathophysiology and the assessment of cardiovascular risk.
Klotho gene was originally identified in renal distal tubular epithelial cell in 1997 [2]. The 130-kD gene encodes a novel protein, namely Klotho, which has been postulated as a regulator of the human aging process. A defect in Klotho gene expression in the mouse result in a phenotype similar to premature human aging, including endothelial dysfunction, progressive atherosclerosis, and shortened lifespan [2,3]. Soluble Klotho, a proteolytic cleavage of the extracellular domain of Klotho, is detectable in the blood, urine, and cerebrospinal fluid [4]. Soluble Klotho has been implicated in anti-apoptotic effects on vascular endothelial cells and cardiovascular protective properties [5,6]. Klotho deficiency has been related to an accelerated development of vascular disorders [7,8]. Recently, several studies show that lower soluble Klotho concentrations are independently associated with a higher likelihood of cardiovascular disease [9-12].
The data is still scarce in the literature with regard to the link between alterations in serum Klotho levels and CVD in Chinese adults. We conducted a cross-sectional study to test the serum Klotho concentrations are associated with coronary artery disease in Chinese adults.
Materials and Methods
Subjects
We enrolled 100 patients who underwent coronary angiography in the Shaanxi Provincial People's Hospital, including 50 patient who diagnosis as coronary heart disease and 50 without coronary heart disease. The patients were less than 70 years of age. A brief medical questionnaire was administered. The exclusion criteria were as follows: severe chronic heart failure, infectious processes within 2 weeks of enrolment, chronic kidney disease, adrenal dysfunction or thyroid dysfunction, or malignancy and patients having treatment with steroids, nonsteroidal anti-inflammatory drugs, or immunosuppressive drugs. This study was approved by the ethics committee of the Shaanxi Provincial People's Hospital. Written informed consent was obtained from all participants.
Coronary angiography was performed by the femoral or radial artery approach according to the Judkins technique and recorded at a rate of 15 frames/s. Significant CHD was defined as at least one major epicardial vessel with >50% stenosis, whereas the control was defined as<50% stenosis within each of the major epicardial vessels.
Biochemical Analyses
All blood samples were obtained from patients in the morning after 8 hours of fasting. Blood samples for the measurement of fasting plasma Klotho concentrations were collected on EDTA-aprotinin tubes and immediately centrifuged at 4 °C, and plasma was collected and stored at −80 °C until analysis. Klotho concentration was determined using a validated sandwich ELISA with a Klotho-specific antibody (Cusabio Biotech Co. Ltd., Wuhan, China). Five plasma samples for Klotho were used to evaluate the intra- and inter-assay coefficients of variation, which ranged from 3.5% to 4.7% (mean, 4.2%) and 4.8% to 6.2% (mean, 5.4%), respectively.
Statistical Analysis
Data were presented as mean ± SD. Differences between biochemical markers were calculated through a one-way analysis of variance (ANOVA). Age, gender, and BMI were adjusted through multivariable analysis. Multiple logistic regression analyses were used to identify the influential parameters on the appearance of CAD. Calculations were performed using SPSS 16.0 for Windows. Probability was assessed using a two-tailed P-value of <0.05 to describe statistical significance.
Results
Profiles of Study Subjects
Table1 summarizes the population characteristics of the patients with CAD group (n=50) and without CAD (n=50). The patients ages ranged similarly from 40 to 68 years (P>0.05). The status of dyslipidemia, DM, obesity and smoking, were not significantly. The frequency of hypertension in CAD group was higher.
Table 1. Baseline Demographic and Clinical Characteristics of the enrolled patients.
Parameter |
All patients (N=100) |
CAD group
(N=50) |
no-CAD group
(N=50) |
p value |
Mean age (year) |
58.2±6.67 |
58.8±6.64 |
57.6±6.72 |
0.363 |
Sex (m/f) |
59/41 |
31/19 |
28/22 |
0.342 |
Body mass index, kg/m 2 |
22.4±3.2 |
23.0±2.1 |
21.9±2.9 |
0.632 |
HbA1c (%) |
6.01±0.75 |
5.88±0.48 |
6.17±0.86 |
0.264 |
urea nitrogen, mmol/L |
5.04±1.53 |
4.73±1.50 |
5.35±1.51 |
0.063 |
Creatinine, mmol/L |
72.4±16.7 |
73.1±18.8 |
71.6±14.5 |
0.652 |
Cystatin c, mmol/L |
1.04±0.44 |
0.98±0.29 |
1.09±0.54 |
0.248 |
Triglycerides, mmol/L |
1.61±0.83 |
1.60±0.77 |
1.61±0.91 |
0.961 |
LDL-cholesterol, mmol/L |
2.24±0.82 |
2.34±0.99 |
2.14±0.59 |
0.234 |
HDL-cholesterol, mmol/L |
1.07±0.24 |
1.10±0.27 |
1.03±0.21 |
0.152 |
Smoking status, (y/n) |
54/46 |
28/22 |
26/24 |
0.421 |
Hypertension, n (%) |
57(57) |
34(68) |
23(46) |
0.021 |
Diabetes mellitus, n (%) |
20(20) |
9(18) |
11(22) |
0.402 |
Serum Klotho concentrations and CAD
As shown in Figure 1, the serum Klotho level from the CAD group was 443.0±29.8pg/ml compared with 616.5±29.4 pg/ml from the non-ELC group, and the difference achieve striking statistical significance. After adjusting for age and gender, the multiple logistic regression analysis revealed that Klotho level was the influential parameter on the appearance of CAD (odds ratio =1.003, 95% confidence interval = 1.002-1.005, p < 0.001), namely a 100 pg/ml reduction of Klotho level was associated with mean 30% increase in CAD incidence.
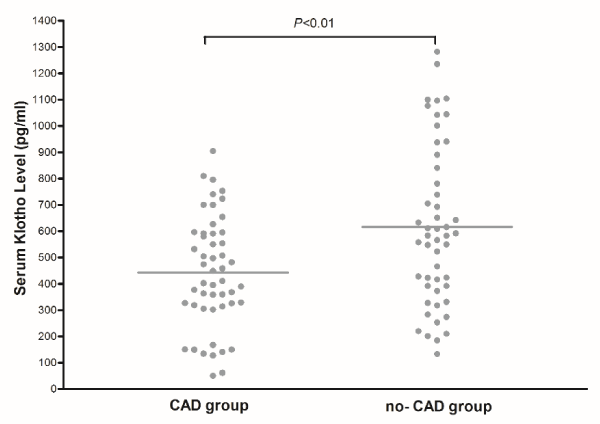
Figure 1. Serum Klotho level from the CAD group was 443.0±29.8pg/ml compared with 616.5±29.4 pg/ml from the non-ELC group.
Discussion
We herein found serum Klotho levels was significantly lower in Chinese patients with CAD. In the logistic regression analysis, a 100 pg/ml reduction of Klotho level was associated with mean 30% increase in CAD incidence.
China is still the biggest country in the world, with more than1.3 billion people, and the morbidity and mortality of CAD increased significantly within the past 30 years. Atherosclerosis is a major source of disability and death in China, and mortality has more than doubled within the past two decades, which exceeds 1 million deaths per year. Therefore, it is becoming more and more urgent for searching new biological systems to explore atherosclerotic pathophysiology and the assessment of cardiovascular risk.
Klotho is an anti-ageing protein that functions in many pathways that govern ageing, which have garnered a lot of attention in vascular biology [13]. Two forms of Klotho, membrane Klotho and secreted Klotho, have been described, exert different functions [7,8,14]. As a soluble co-receptor, secreted Klotho could participate in the regulation of nitric oxide production in the endothelium and maintain endothelial integrity by mediating vascular endothelial growth factor (VEGF)-induced internalization [15-18]. Klotho also could protect against endothelial cell apoptosis through the mitogen-activated protein kinase pathway and reduces tumor necrosis factor αinduced nuclear factor κB activation [19-21]. The animal studies present that the vascular phenotype of Klotho deficiency is very similar to both human ageing and “accelated” ageing, such as atherosclerosis, vascular calcifications, and endothelial dysfunction [2,22,23]. Furthermore, recombinant soluble klotho administration extends the life span and ameliorate the premature aging-related phenotype.
Recently, several studies have shown that deficiency of Klotho may be an important factor in the development of CVD. Navarro-González, et al. [9] performed a cross-sectional study which show patients with CAD present lower soluble concentrations of Klotho and reduced levels of Klotho gene expression in the vascular wall. The “Aging in the Chianti Area” (InCHIANTI) study including 1023 found that higher plasma klotho concentrations are independently associated with a lower likelihood of having cardiovascular disease in community-dwelling adults [10]. The present study also found serum Klotho levels were significantly descended in the CAD group, which is consistent with previous studies.
The strength of this study is that the patients were recruited by age-matching, chronic kidney disease was excluded, and the multiple logistic regression analysis was also performed, thus confounding due to these exposures should be minimized. Further, we used coronary angiography for the diagnosis of CHD. Meanwhile, a few limitations of the current analysis are worth discussing. The sample size was small, and further studies are required to validate our findings in a larger and more diverse sample.
Conclusion
In conclusion, this study shows the Chinese patients with lower Klotho concentrations have a higher risk of coronary artery disease. Lower Klotho may represent a potential risk factors for coronary artery disease and sheds some new light for therapeutic interventions in the future.
Acknowledgements
This study was supported by Natural Science Basic Research Plan of Shaanxi Province (No.2017KJXX-70), Special Financial Grant from China Postdoctoral Science Foundation (No. 2017T100789), and Natural Science Foundation of Shaanxi Province (No. 2016JM8025).
References
- 1. Mathers CD, Loncar D (2006) Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med 3: e442. [Crossref]
- 2. Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, et al. (1997) Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 390: 45-51. [Crossref]
- 3. Nagai R, Saito Y, Ohyama Y, Aizawa H, Suga T, et al. (2000) Endothelial dysfunction in the klotho mouse and downregulation of klotho gene expression in various animal models of vascular and metabolic diseases. Cell Mol Life Sci 57: 738-746. [Crossref]
- 4. Martin-Nunez E, Donate-Correa J, Muros-de-Fuentes M, Mora-Fernandez C, NavarroGonzalez JF (2014) Implications of Klotho in vascular health and disease. World J Cardiol 6: 1262-1269. [Crossref]
- 5. Saito Y, Yamagishi T, Nakamura T, Ohyama Y, Aizawa H, et al. (1998) Klotho protein protects against endothelial dysfunction. Biochem Biophys Res Commun 248: 324-329. [Crossref]
- 6. Saito Y, Kuroo M, Nabeshima Y, Nagai R (1999) The protective role of Klotho gene on vascular endothelium. Nihon Rinsho 57: 1514-1518.
- 7. Xu Y, Sun Z (2015) Molecular basis of Klotho: from gene to function in aging. Endocr Rev 36: 174-193. [Crossref]
- 8. Kim JH, Hwang KH, Park KS, Kong ID, Cha SK (2015) Biological Role of Anti-aging Protein Klotho. J Lifestyle Med 5: 1-6. [Crossref]
- 9. Navarro-Gonzalez JF, Donate-Correa J, de Fuentes MM, Perez-Hernandez H, Martinez-Sanz R, et al. (2014) Reduced Klotho is associated with the presence and severity of coronary artery disease. Heart 100: 34-40. [Crossref]
- 10. Semba RD, Cappola AR, Sun K, Bandinelli S, Dalal M, et al. (2011) Plasma klotho and cardiovascular disease in adults. J Am Geriatr Soc 59: 1596-1601. [Crossref]
- 11. Su XM, Yang W (2014) Klotho protein lowered in elderly hypertension. Int J Clin Exp Med 7: 2347-2350. [Crossref]
- 12. Keles N, Caliskan M, Dogan B, Keles NN, Kalcik M, et al. (2015) Low Serum Level of Klotho Is an Early Predictor of Atherosclerosis. Tohoku J Exp Med 237: 17-23. [Crossref]
- 13. Donate-Correa J, Mora-Fernandez C, Martinez-Sanz R, Muros-de-Fuentes M, P�rez H, et al. (2013) Expression of FGF23/KLOTHO system in human vascular tissue. Int J Cardiol 165: 179-183. [Crossref]
- 14. Donate-Correa J, Martin-Nunez E, Mora-Fernandez C, Muros-de-Fuentes M, Perez-Delgado N, et al. (2015) Klotho in cardiovascular disease: Current and future perspectives. World J Biol Chem 6: 351-357. [Crossref]
- 15. Mencke R, Hillebrands JL (2017) The role of the anti-ageing protein Klotho in vascular physiology and pathophysiology. Ageing Res Rev 35: 124-146. [Crossref]
- 16. Kusaba T, Okigaki M, Matui A, Murakami M, Ishikawa K, et al. (2010) Klotho is associated with VEGF receptor-2 and the transient receptor potential canonical-1 Ca2+ channel to maintain endothelial integrity. Proc Natl Acad Sci U S A 107: 19308-19313. [Crossref]
- 17. Richter B, Haller J, Haffner D, Leifheit-Nestler M (2016) Klotho modulates FGF23mediated NO synthesis and oxidative stress in human coronary artery endothelial cells. Pflugers Arch 468: 1621-1635. [Crossref]
- 18. Carracedo J, Buendia P, Merino A, Madue?o JA, Peralbo E, et al. (2012) Klotho modulates the stress response in human senescent endothelial cells. Mech Ageing Dev 133: 647-654. [Crossref]
- 19. Maekawa Y, Ohishi M, Ikushima M, Yamamoto K, Yasuda O, et al. (2011) Klotho protein diminishes endothelial apoptosis and senescence via a mitogen-activated kinase pathway. Geriatr Gerontol Int 11: 510-516. [Crossref]
- 20. Wang Y, Kuro-o M, Sun Z (2012) Klotho gene delivery suppresses Nox2 expression and attenuates oxidative stress in rat aortic smooth muscle cells via the cAMP-PKA pathway. Aging Cell 11: 410-417. [Crossref]
- 21. Maekawa Y, Ishikawa K, Yasuda O, Oguro R, Hanasaki H, et al. (2009) Klotho suppresses TNF-alpha-induced expression of adhesion molecules in the endothelium and attenuates NF-kappaB activation. Endocrine 35: 341-346. [Crossref]
- 22. Lim K, Lu TS, Molostvov G, Lee C, Lam FT, et al. (2012) Vascular Klotho deficiency potentiates the development of human artery calcification and mediates resistance to fibroblast growth factor 23. Circulation 125: 2243-2255. [Crossref]
- 23. Chen TH, Kuro-O M, Chen CH, Sue YM, Chen YC, et al. (2013) The secreted Klotho protein restores phosphate retention and suppresses accelerated aging in Klotho mutant mice. Eur J Pharmacol 698: 67-73. [Crossref]

