Abstract
Objective: To explore the value of ultrasound-guided radiofrequency ablation combined with puncture and aspiration in the intrauterine treatment of fetal sacrococcygeal cystic and solid teratoma.
Methods:The authors performed ultrasound-guided radiofrequency ablation combined with puncture and aspiration for the treatment of fetal sacrococcygeal cystic and solid teratoma, and reviewed the literature on the intrauterine treatment options for fetal sacrococcygeal teratoma.
Results: Ultrasound-guided radiofrequency ablation combined with puncture and aspiration is a safe and effective method for the treatment of fetal sacrococcygeal cystic-solid teratoma.
Conclusions: The proposed treatment for fetal sacrococcygeal cystic-solid teratoma was successful for this kind of lesion.
Keywords
Sacrococcygeal teratoma; Intrauterine fetal treatment; Ultrasound; Radiofrequency ablation; Puncture and aspiration
Introduction
Fetal sacrococcygeal teratoma (SCT) is a rare disease but one of the most common fetal tumors. It is a germ cell tumor located in the presacral region, and it originates from multipotent stem cells within the Hensen node[1]. Furthermore, it is a tumor that occurs in approximately 1 in 35,000 to 40,000 live births, and female fetuses account for 80% of cases[2]. Although the majority of postnatally diagnosed cases have good prognosis, the mortality rate for prenatally diagnosed SCT is still high, with a reported value of 33% to 53%. The causes of intrauterine death are mostly cardiac failure or edema, while those of postnatal death are predominantly premature delivery or tumor rupture and bleeding[3,4]. Therefore, SCT diagnosed prenatally with the risk of cardiac failure, edema or tumor rupture needs timely intervention. At present, the intervention measures mainly include prenatal intervention and postpartum surgical treatment. Prenatal intervention also involves open surgery and intrauterine minimally invasive treatment. Magdalena Litwińska,et al.reviewed cases related to intrauterine minimally invasive treatment from 1996 to 2018 and concluded that the intrauterine minimally invasive treatments for teratoma containing solid components in the study period mainly included ultrasound-guided radiofrequency ablation and ultrasound-guided laser ablation[5]. This paper reports a successful case of ultrasound-guided radiofrequency ablation combined with puncture and aspiration for the intrauterine treatment of fetal sacrococcygeal cystic-solid teratoma,and discusses the application value of this treatment approach based on our experience and relevant literature review.
Case report
A 27-year-old female patient, G1P0A0L0, who was examined at the local hospital at 12 weeks of gestation, showed an NT value of 1.6 mm with no obvious abnormalities in the fetus. At 25 weeks and 2 days of gestation, a cystic-solid mass with a size of 6.0*4.8*3.2 cm was detected in the sacrococcygeal region of the fetus in the same hospital, and no other obvious abnormalities were observed. Then, MRI was performed and "fetal sacrococcygeal teratoma" was considered. The patient was examined at 26 weeks of gestation and the size of the fetal sacrococcygeal mass was 7.0*6.0*5.6 cm. Since the mass continued to grow, the patient came to our hospital for further treatment at 27+1 weeks of gestation. Ultrasound examination was performed, which indicated that the size of the fetal sacrococcygeal mass was 7.1*7.0*6.2 cm, it was regular in shape and cystic-solid, some areas of the dorsal part were solid, and the range of the solid part was about 5.3*0.9 cm. There was a small blood flow signal in the solid part of the mass; the amniotic fluid was normal, and the placenta was located in the posterior wall of the uterus (Figure1). The amniotic fluid DNA test results showed no increase/deletion of the chromosome copy number.
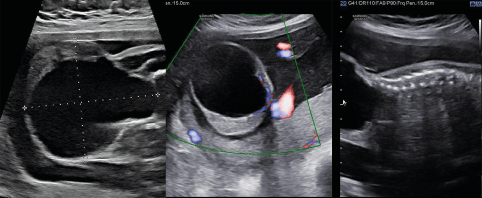
Figure 1. The pregnant woman received obstetric ultrasound in our hospital at 27+1 weeks of gestation. The mass was mainly located on the exterior area (sacrococcygeal region), and only a small part of it was located on the presacral area. It was cystic-solid with a small portion of solid components in it, and blood flow signals could be seen in the solid part
A previous study reported that a mass larger than the biparietal diameter indicates poor fetal prognosis[6]. Considering the progressive enlargement of the fetal sacrococcygeal mass in our case, its growth rate had been faster than the growth rate of the biparietal diameter (BPD=7.0 cm, maximum mass diameter= 7.1 cm). After a joint discussion of obstetrical fetal medical experts and ultrasound medical experts in our hospital, it was concluded that the fetus was at risk of cardiac failure, edema, dystocia, tumor rupture, and death. Following full discussion with the pregnant woman and her family members, we decided to implement intrauterine intervention to reduce the possibility of serious complications and prevent adverse outcomes as much as possible, so that the fetus could safely grow to term for postpartum surgery.
Based on comprehensive consideration, we decided to perform ultrasound-guided radiofrequency ablation combined with puncture and aspiration for fetal sacrococcygeal teratoma. We conducted careful preoperative preparations. Firstly, through radiofrequency ablation on isolated pig liver, a pre-experiment was performed to find out the relationship between ablation power, ablation time and ablation range, in order to determine surgical ablation power, ablation time and ablation needle type. Secondly, the treatment plan was designed, and it was considered that if the fluid is drawn out before the ablation, the mass may bleed due to the reduced pressure in the capsule, and if the fluid in the capsule is thick and cannot be drawn out, there may be unpredictable risks during the operation. Therefore, we decided to adopt the surgical strategy of first ablating the solid part of the capsule wall, then drawing out the fluid, and finally ablating the residual solid part.
Surgical procedure
A preoperative intravenous drip of magnesium sulfate was administered to reduce uterine sensitivity and prevent uterine contraction and premature delivery. Infections were prevented by intravenous drip of cefuroxime sodium. During the operation, the pregnant woman was injected with lidocaine for local infiltration anesthesia layer by layer. Then, to anesthetize the fetus, intramuscular injections of 0.1 mg of vecuronium bromide and 0.045 mg of fentanyl were given to the buttocks of the fetus. An RF generator (Cool-tip RF system: Covidien, Bolder, USA/VIVA RF system: STARmed, Goyang, Korea) and an 18-gauge, 1.0 cm active-tip internally cooled electrode (Well-Point RF Electrode: STARmed, Goyang, Korea) were used, and the ablation power was set to 80 W. We used an ultrasound machine (GE Healthcare-LOGIQ E9) to guide the insertion of an ablation needle percutaneous into the tumor. We first ablated the solid part of the capsular wall, then used a 23-gauge 9 cm puncture needle to draw out 100 mL of thin milky yellow liquid, and proceeded to ablate the solid part of the remaining capsular wall (Figure 2). The surgery lasted for 40 minutes, including 13 minutes of radiofrequency ablation. During the operation, the patient had no obvious adverse reactions, and the fetal heart monitoring exhibited no obvious abnormalities. The fetus and pregnant mother were re-examined on the first day after operation. The sacrococcygeal mass of the fetus was significantly reduced, showing a size of 6.0*5.2*4.5 cm. The volume of the preoperative mass was 160 mL, the current mass volume was 73 mL, and the nodule reduction rate was 54%. There was no obvious blood flow signal in the solid part of the mass, and the health status of the mother and fetus were normal. They were re-examined one month after the operation, and the mass gradually increased to 7.6*6.8*5.3 cm, close to the preoperative size. A few blood flow signals were detected in the solid part of the mass, and the biparietal diameter of the fetus was 8.2 cm. Considering that the fetus had been at 32 weeks of gestation at that time, with normal growth and development and no obvious intrauterine growth retardation, after comprehensive evaluation, we decided to carry out ultrasound-guided puncture and aspiration treatment only for the fetal sacrococcygeal cystic-solid mass, hoping to reduce the tumor volume and control its growth rate, such that the fetus could grow safely to term and ensure the safety of the mother and fetus during delivery. Then, at 32+1 weeks of gestation, ultrasonic-guided percutaneous puncture was performed again and 110 ml of thin yellow liquid was drawn out. The fetal heart was normal during the operation and the pregnant mother had no obvious adverse reactions. MRI examination of the fetus and pregnant mother after operation showed that the size of the fetal sacrococcygeal mass was 6.0*5.8*3.8cm (Figure 3).The volume of the preoperative mass was 142.4mL, the current mass volume was 68.8 mL, and the nodule reduction rate was 51.7%.
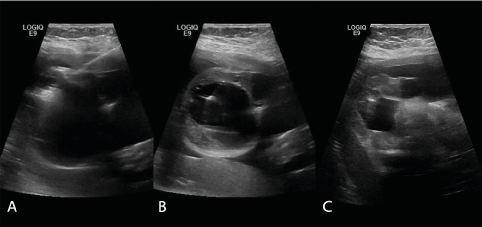
Figure 2. Radiofrequency ablation combined with puncture and aspiration for the sacrococcygeal mass: A. Radiofrequency ablation; B. Puncture and aspiration; C. The volume of the mass was reduced after the operation
Two weeks after the second surgery (34+1 weeks of gestation), the fetus and the pregnant mother were re-examined. The mass was 7.1*6.8*6.6 cm, with a biparietal diameter of 8.7 cm, and there were a few blood flow signals in the solid part of the mass. Then, the patients were followed up and waited for elective cesarean section. The trend of the mass size during the follow-up was shown in Figure 4. Caesarean section was performed at 38 weeks of gestation and a baby girl weighing 2910 g was successfully delivered. The neonate had Apgar scores of 10 and 10 at 1 and 5 mins, respectively. A mass of about 6.0*6.0*5.0cm could be seen in the sacrococcygeal region of the baby (Figure 5). The skin on the surface of the mass was smooth without damage and ulceration. The baby had normal cardiac function (LVEF 66%). She underwent complete resection of sacrococcygeal mass on the 7th day after birth. The postoperative pathological results were as follows: (sacrococcygeal) cystic-solid mature teratoma (the gross specimen and pathology are shown in Figure 5).
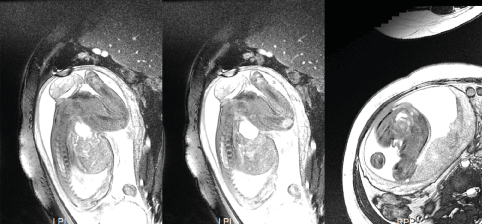
Figure 3. MRI of the fetus (after the second surgery)
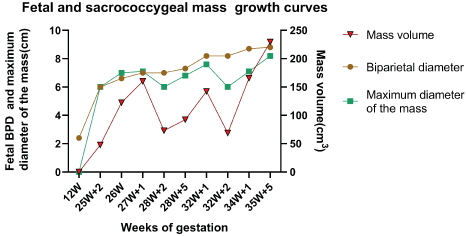
Figure 4. Growth curves of the fetus and the sacrococcygeal mass
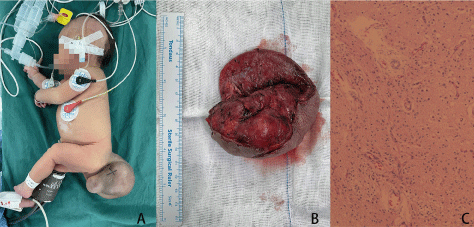
Figure 5. A. Sacrococcygeal mass of the neonate (7th day after birth, before surgical resection); B. The gross specimen of this sacrococcygeal mass; C. Pathological result: Cystic-solid mature teratoma
Discussion
The American Academy of Pediatrics (AAP) classifies sacrococcygeal teratoma into four types based on the site of the tumor: Type I are predominantly external (sacrococcygeal) tumors with only a minimal presacral component; Type II are tumors presenting externally but with a significant intrapelvic extension; Type III are externally apparent tumors but the predominant mass is pelvic and extends into the abdomen; Type IV are presacral with no external presentation[7]. According to its internal components, the tumor can be divided into three categories: solid, cystic and cystic-solid. Our case belonged to Type I. The mass was cystic-solid, most of which consisted of cystic components with a small proportion of solid components. Although most fetal sacrococcygeal teratomas are benign tumors, those that are prenatally detected tend to have poor prognosis. The causes of death are mostly fetal high-output cardiac failure and edema, while for some it is tumor rupture, hemorrhage, premature delivery and dystocia, or the erosion of surrounding tissue by malignant teratoma[8].
Cystic or hypovascular teratoma has little impact on the fetus because of its slow growth. However, some tumors with solid components grow rapidly, especially those with rich blood supply, as an arteriovenous shunt (AVS) easily leads to fetal high-output cardiac failure and edema, and can also result in "mirror syndrome" in pregnant women, manifested as edema and hypertension[9]. Once fetal edema occurs, fetal mortality will be almost 100% without timely intervention[9,10]. Therefore, intrauterine treatment is particularly important in prenatally diagnosed fetal sacrococcygeal teratoma.
At present, there are five main schemes for intrauterine treatment: (i) Ultrasound-guided percutaneous needle drainage of cystic sacrococcygeal teratomas[11]; (ii) Ultrasound-guided alcohol sclerotherapy[12–14]; (iii) Open fetal surgery[15]; (iv) Ultrasound-guided radiofrequency ablation[16]; (v) Laser ablation: a. Intrauterine endoscopic laser ablation of the feeding vessels of the fetal sacrococcygeal teratoma[17]; b. Ultrasound-guided percutaneous laser ablation of sacrococcygeal teratoma[18].
Most fetal cystic teratomas have good prognosis, but when their diameter is too large, they easily cause dystocia, tumor rupture and bleeding. When they are classified as grade IV or their volume is too large, they will also compress the fetal bladder and bilateral ureters, resulting in urethral obstruction and serious hydronephrosis, which will affect the development of the urinary system. At this time, ultrasound-guided puncture drainage can be used to alleviate the pressure caused by the tumor and reduce the incidence of complications[19,20]. For teratoma with solid components, five cases of ultrasound-guided alcohol sclerotherapy have been reported in the literature, and only one patient survived [12–14]. Due to the teratogenic effect of alcohol on the fetus, no similar literature has been reported since these cases.
Although open fetal surgery can effectively improve the symptoms of fetal heart failure and edema[15], the reported postoperative fetal survival rates are low at 43%-55%[8,21]. Currently, this surgical technique is highly controversial. Intrauterine endoscopic laser ablation of the feeding vessels is less effective due to the limitation that the intrauterine endoscope cannot accurately find the feeding vessels, hence the postoperative survival rate is only 33% [12,17,22]. Ultrasound-guided radiofrequency ablation and ultrasound-guided laser ablation both have a satisfactory therapeutic effect. For these techniques, 29 cases have been reported (Table 1)[5,14,16,18,22–26], and their postoperative survival rates were 64% (9/14) and 47% (7/15), respectively. These two treatments are currently adopted by most doctors. The postoperative survival rate of fetuses with these two treatments was 50% (11/22) when combined with heart failure, 33.3% (3/9) when combined with edema, and 71.4% (5/7) when not combined with heart failure and edema.
Table 1. The data of ultrasound-guided radiofrequency ablation and ultrasound-guided laser ablation
Reference (first author) |
Therapeutic intervention |
Gestational weeks |
Heart failure |
Edema |
Outcome |
Van Mieghem[22] |
radiofrequency ablation |
26 |
yes |
yes |
alive |
Lee[23] |
radiofrequency ablation |
25 |
yes |
no |
alive |
Lee[23] |
radiofrequency ablation |
31 |
yes |
no |
alive |
Paek[16] |
radiofrequency ablation |
18 |
yes |
no |
alive |
Lam[24] |
radiofrequency ablation |
18 |
yes |
yes |
death |
Paek[16] |
radiofrequency ablation |
20 |
yes |
yes |
death |
Van Mieghem[22] |
radiofrequency ablation |
22 |
yes |
yes |
death |
Ibrahim[25] |
radiofrequency ablation |
19 |
no |
no |
alive |
Lee[23] |
radiofrequency ablation |
23+30 |
no |
no |
alive |
Lee[23] |
radiofrequency ablation |
23 |
no |
no |
alive |
Lee[23] |
radiofrequency ablation |
31 |
no |
no |
alive |
Paek[16] |
radiofrequency ablation |
19 |
no |
no |
alive |
Paek[16] |
radiofrequency ablation |
22 |
no |
no |
termination |
Lee[23] |
radiofrequency ablation |
20+22 |
no |
no |
neonatal death |
Sananes[14] |
laser ablation |
21+24+28 |
yes |
no |
alive |
Ding[26] |
laser ablation |
22 |
yes |
no |
alive |
Litwińska[5] |
laser ablation |
20 |
yes |
no |
alive |
Litwińska[5] |
laser ablation |
21+24 |
yes |
no |
alive |
Litwińska[5] |
laser ablation |
23+27 |
yes |
no |
alive |
Sananes[14] |
laser ablation |
22+27 |
yes |
yes |
alive |
Van Mieghem[22] |
laser ablation |
26+27 |
yes |
yes |
alive |
Ruano[18] |
laser ablation |
24 |
yes |
yes |
death |
Van Mieghem[22] |
laser ablation |
17 |
yes |
no |
death |
Litwińska[5] |
laser ablation |
20+22 |
yes |
no |
death |
Sananes[14] |
laser ablation |
24 |
yes |
yes |
death |
Sananes[14] |
laser ablation |
21+22 |
yes |
yes |
neonatal death |
Litwińska[5] |
laser ablation |
19 |
yes |
no |
neonatal death |
Litwińska[5] |
laser ablation |
19+20 |
yes |
no |
neonatal death |
Litwińska[5] |
laser ablation |
23+24 |
yes |
no |
neonatal death |
However, both methods still have the potential to cause fetal death. Two main causes of fetal death were reported when these two treatments were used: (i) When most of the tumor is ablated, it may cause entry of tissue thromboplastins into the bloodstream, resulting in activation of the coagulation cascade that leads to coagulation dysfunction in the fetus and ultimately to disseminated intravascular coagulation (DIC)[27]. (ii) The occurrence of postoperative premature rupture of membranes[23]. Therefore, restricted ablation may be necessary.
In our case, the fetal sacrococcygeal mass grew rapidly. No mass was found at 12 weeks of gestation, but the diameter of the sacral caudal masses reached 7.1 cm at 27 weeks of gestation, and the growth rate of its diameter was faster than that of its biparietal diameter. It was reported that the fetal mortality rate after performing intrauterine intervention when the fetus has symptoms of cardiac failure or edema would be as high as 50%[5,14,16,18,22–24,26] Therefore, even though the fetus in our case had no symptoms of cardiac failure and edema at that time, we decided to carry out intrauterine intervention on the fetus to slow down the tumor growth rate and reduce the tumor volume, in order to reduce the possibility of tumor related complications and strive for sufficient time for the fetus to grow safely to term and remove the mass after birth.
In our case, the tumor volume was significantly reduced after the first operation, with a reduction rate of 54.4%. However, one month after surgery, the tumor gradually grew to a similar size as before. We considered that this might be caused by the shorter ablation time due to safety considerations during the first operation. Meanwhile, the biparietal diameter of the fetus after surgery was already larger than the tumor diameter, indicating that the first operation effectively controlled the growth rate of the tumor to a certain extent. Considering that the gestational age of the pregnant woman at that time was 32 weeks, the growth and development of the fetus was normal, and the pregnancy could be terminated at any time. To reduce the risk of surgery, we decided to only perform puncture and aspiration for the mass in the second operation. This method effectively reduced the tumor volume and slowed down its growth rate, and also ensured the safety of the pregnant mother and fetus during delivery. The baby's cardiac function was normal after birth, and the tumor was completely removed on the 7th day after birth. During the recent follow-up, the baby was in good health.
Currently, there is no unified standard for the indications and treatment methods of intrauterine treatment of fetal sacrococcygeal teratoma. Most fetal medical experts opt to carry out intrauterine treatment only when the fetus has symptoms of cardiac failure and edema, but this will lead to poor prognosis, and result in a postoperative fetal survival rate of only 50%[5,14,16,18,22-24,26]. The successful implementation of the proposed surgical approach in this case suggests that early intrauterine intervention for fast-growing fetal cystic-solid tumors may be a better option before the fetus develops symptoms of cardiac failure or edema. The method of radiofrequency ablation of the solid part of the tumor to reduce its activity and the aspiration of its cystic fluid to reduce its volume provide a safe and effective alternative for the treatment of this kind of tumor in the future.
Conclusions
Fetal sacrococcygeal teratoma is the most common tumor of the fetus. Although most cases are benign, the prognosis of fetuses with prenatally diagnosed teratoma is poor. Such fetuses often have serious complications such as cardiac failure, edema, polyhydramnios, severe hydronephrosis, tumor rupture and bleeding, which need timely intervention. Although various methods of intrauterine therapy exist, these are not perfect and their postoperative survival rate is not sufficient. In this paper, we have reported a new approach to treat fetal sacrococcygeal cystic-solid teratoma, which was successful for this kind of lesion.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Author contributions
Guarantors of integrity of entire study, J.Z.; study concepts/study design or data acquisition or data analysis/interpretation, J.Z., Q.G.Z., Q.L.; manuscript drafting or manuscript revision for important intellectual content, J.Z.,Q.G.Z.,Q,L.,Y.T.H., M.Y.W.,X.F.F.; approval of final version of submitted manuscript, J.Z., Q.L., Q.G.Z.; agrees to ensure any questions related to the work are appropriately resolved, all authors; literature research, J.Z.,Q.G.Z.,Q.L.,Y.T.H.,M.Y.W; clinical studies,J.Z.,Q.G.Z.,Q.L.,S.S.L.,L.C.W.;manuscript editing, all authors.
Funding
Not Applicable.
Acknowledgments
Not Applicable.
Ethics approval and consent to participate
The study was approved by the ethics review board of Department of Ultrasound, Women’s Hospital, Zhejiang University School of Medicine (IRB-20220017-R) in accordance with the Declaration of Helsinki. Written informed consent was obtained from all individual patients included in the study.
Consent for publication
All authors have consented to the publication of the manuscript.
Data Availability Statement
Data generated or analyzed during the study are available from the corresponding author by request.
References
- Brace V, Grant SR, Brackley KJ, Kilby MD, Whittle MJ (2000) Prenatal diagnosis and outcome in sacrococcygeal teratomas: a review of cases between 1992 and 1998. PrenatDiagn20: 51–55. [Crossref]
- Graf JL, Albanese CT (2003) Fetal Sacrococcygeal Teratoma. World Journal of Surgery 27: 84–86. [Crossref]
- Holterman A-X, Filiatrault D, Lallier M, Youssef S (1998) The natural history of sacrococcygeal teratomas diagnosed through routine obstetric sonogram: A single institution experience. J PediatrSurg33: 899-903. [Crossref]
- Hedrick HL, Flake AW, Crombleholme TM, Howell LJ, Johnson MP, et al. (2004) Sacrococcygeal teratoma: prenatal assessment, fetal intervention, and outcome. J PediatrSurg39: 430-438. [Crossref]
- Litwińska M, Litwińska E, Janiak K, Piaseczna-Piotrowska A, Szaflik K (2020) Percutaneous Intratumor Laser Ablation for Fetal Sacrococcygeal Teratoma. FetalDiagnTher47: 138–144. [Crossref]
- Vergnes P, Horovitz J, Mauge B, Lamireau T, Colombani JF, et al. (1991) Sacrococcygeal teratomas in antenatal diagnosis. J GynecolObstetBiolReprod (Paris)20: 633-642.
- Peter Altman R, Randolph JG, Lilly JR (1974) Sacrococcygeal teratoma: American Academy of Pediatrics Surgical Section survey—1973. J PediatrSurg9: 389-398. [Crossref]
- Lan Z, Xiaodong W, Haiyan Y (2019) Research progress of fetal sacrococcygeal teratoma. Journal of Practical Obstetrics and Gynecology 35: 24-26.
- Langer JC, Harrison MR, Schmidt KG, Silverman NH, Anderson RL, et al. (1989) Fetal hydrops and death from sacrococcygeal teratoma: Rationale for fetal surgery. American Journal of Obstetrics and Gynecology160: 1145-1150.
- Grethel EJ, Wagner AJ, Clifton MS, Cortes RA, Farmer DL, et al. (2007) Fetal intervention for mass lesions and hydrops improves outcome: a 15-year experience. J PediatrSurg42: 117–123. [Crossref]
- Kay S, Khalife S, Laberge JM, Shaw K, Morin L, et al. (1999) Prenatal percutaneous needle drainage of cystic sacrococcygeal teratomas. J PediatrSurg34: 1148–1151.
- Makin EC, Hyett J, Ade-Ajayi N, Patel S, Nicolaides K, et al. (2006) Outcome of antenatally diagnosed sacrococcygeal teratomas: single-center experience (1993-2004). J PediatrSurg41: 388–393. [Crossref]
- Perrotin F, Herbreteau D, Machet MC, Potin J, Lardy H, et al. (2006) OP06.20: In utero Doppler ultrasound-guided embolization for the treatment of a large, vascular sacrococcygeal teratoma causing fetal hydrops. Ultrasound ObstetGynecol28: 458–459.
- Sananes N, Javadian P, Schwach Werneck Britto I, Meyer N, Koch A, et al. (2016) Technical aspects and effectiveness of percutaneous fetal therapies for large sacrococcygeal teratomas: cohort study and literature review: Laser for sacrococcygeal teratoma. Ultrasound ObstetGynecol47: 712–719. [Crossref]
- Graf JL, Albanese CT, Jennings RW, Farrell JA, Harrison MR (2000) Successful fetal sacrococcygeal teratoma resection in a hydropicfetus. J PediatrSurg35: 1489-1491.
- Paek BW, Jennings RW, Harrison MR, Filly RA, Tacy TA, et al. (2001) Radiofrequency ablation of human fetal sacrococcygeal teratoma. Am J ObstetGynecol184: 503–507. [Crossref]
- Hecher K, Hackelöer BJ (1996) Intrauterine endoscopic laser surgery for fetal sacrococcygeal teratoma.Lancet 347: 470. [Crossref]
- Ruano R, Duarte S, Zugaib M (2009) Percutaneous laser ablation of sacrococcygeal teratoma in a hydropicfetus with severe heart failure--too late for a surgical procedure? FetalDiagnTher25: 26–30. [Crossref]
- Kay S, Khalife S, Laberge JM, Shaw K, Morin L, et al. (1999) Prenatal percutaneous needle drainage of cystic sacrococcygeal teratomas. J PediatrSurg34: 1148-1151.
- Garcia AM, Morgan III WM, Bruner JP (1998) In utero Decompression of a Cystic Grade IV Sacrococcygeal Teratoma. FetalDiagnTher13: 305–308. [Crossref]
- Hirose S, Farmer DL (2003) Fetal surgery for sacrococcygeal teratoma. Clin Perinatol30: 493–506. [Crossref]
- Van Mieghem T, Al-Ibrahim A, Deprest J (2014) Minimally invasive therapy for fetal sacrococcygeal teratoma: case series and systematic review of the literature. Ultrasound ObstetGynecol43: 611-619. [Crossref]
- Lee MY, Won HS, Hyun MK, Lee HY, Shim JY, et al. (2011) Perinatal outcome of sacrococcygeal teratoma. PrenatDiagn31: 1217–1221. [Crossref]
- Lam YH, Tang MHY, Shek TWH (2002) Thermocoagulation of fetal sacrococcygeal teratoma. PrenatDiagn 22: 99-101. [Crossref]
- Ibrahim D, Ho E, Scherl SA, Sullivan CM (2003) Newborn with an open posterior hip dislocation and sciatic nerve injury after intrauterine radiofrequency ablation of a sacrococcygeal teratoma. J PediatrSurg38: 248–250.
- Ding J, Chen Q, Stone P (2010) Percutaneous laser photocoagulation of tumour vessels for the treatment of a rapidly growing sacrococcygeal teratoma in an extremely premature fetus. J MaternFetal Neonatal Med 23: 1516-1518. [Crossref]
- Murphy JJ, Blair GK, Fraser GC (1992) Coagulopathy associated with large sacrococcygeal teratomas. J PediatrSurg27: 1308-1310. [Crossref]





