An association between increased rates of childhood leukemia and proximity to high voltage electricity power transmission lines was found in a 2005 study by Draper et al. More recently, this study was extended to include a further period of time and with an enlarged dataset by Bunch et al 2014, the result being that there was no longer any overall statistically significant effect for the whole period. Using the data from these two studies the trend in Relative Risk with fallout dose is examined for five sub-periods 1962-69, 1970-79, 1980-89, 1990-99 and 2000-2008. The effect turns out to be significantly associated with the levels of radioactive fallout from atmospheric testing (Chi-square for trend = 7.6; p = 0.006). A non-linear association between the Relative Risk and the Fallout doses is robust, R2 = 0.955; F-statistic 65.6; p = 0.004. Fews et al. (1999) raised the issue of the concentration of airborne radioactive particles near power lines and it is suggested that the observed trend in Relative Risk with time supports a hypothesis in which the inhalation of radioactive particulates from fallout may be the cause, or related to the cause of the effect. The hypothesis is extended to a general discussion of child leukemia and radiation which implicates particulates.
The issue of child cancer and high voltage power lines has been an area of controversy since 1979 when Wertheimer and Leeper reported an association with low frequency (wiring) electromagnetic (EM) radiation [1]. A 2005 large case-control study by the Childhood Cancer Research Group (CCRG) in Oxford [2] demonstrated a modest but statistically significant excess of child leukaemia 0-14 in those living less than 600m from a high voltage power line. For those children within 200m the relative risk (RR) was 1.64. This Draper et al study [2] covered the period 1962-95 and involved 9700 children with leukemia and the same number of controls. The powerlines examined were the 400 and 275kV lines. An extension of this large case control study by Bunch et al, was published in the British Journal of Cancer on 6th February 2014 [3]. The Bunch et al 2014 study [3] increased the number of children with leukaemia to 16620 by extending the period from 1962 to 2008 and adding Scotland to England and Wales. The authors found that over the whole period and for all the children the effect declined over time from a relative risk of 4.5 in the 60s to 0.71 in the 2000s. They conclude that “a risk declining over time” cannot arise from any physical effect of the powerlines and is more likely to be a result of changing populations of those living near powerlines.
However, there is a potential explanation. Since childhood leukemia has been associated in many studies [4-10] with proximity to nuclear sites, in particular fuel reprocessing sites like Sellafield in the UK [10] it seems possible that radioactive exposures of some kind may be reasonably investigated as a cause. The largest injection of radioactivity into the global environment was the atmospheric nuclear testing that was carried out in the period 1950-1963 when the fallout contamination peaked in 1959-63. However, studies using data after 1960 have not reported any significant large excess risk of child leukemia trends [11,12]. This may be because the same period of high fallout exposures saw an increase in infant mortality from all causes, an effect which is capable of quenching increases of child leukemia at higher doses due to the death of pre-leukemic individuals due to immune system incompetence [13]. On the other hand, within national data, child leukemia at the time of the peaks in weapons fallout has been associated with fallout exposure using rainfall as a surrogate. [14]. Since it has been established that high voltage power lines concentrate particles [15] it was of interest to see if the variation in the power line and child leukemia association could be explained by the trend in fallout over the period of the power line studies. Clearly, although all individuals were exposed to fallout over the period of testing, these case control studies [2, 3] are comparing leukemia rates in children and their parents exposed to fallout particles concentrated by the electrostatic effects of power lines with those not so exposed.
The data for the examination of the trend in child leukemia effect is available from the two studies of the Childhood Cancer Research Group (CCRG), in 2005 [2] and 2014 [3]. The data from the Bunch et al 2014 study [3] enables a straightforward analysis of any effect. The data, cases and controls were grouped into 5 periods. 1962-1969, 1970-1979, 1980-1989, 1990-2000 and 2000-2008. The numbers are given in table 1 together with the Relative Risks reported by Bunch et al 2014. The association between the trend in childhood leukemia and the trend in radioactive fallout was examined.
Table 1: Relative Risk (by regression) for case-control study of childhood leukemia 0-14 (data from Bunch et al 2014 [3]). * adjusted by the authors for higher control numbers.
Period |
RR leukemia
0-200m |
Cases
0-200m |
Controls
0-200m |
Number of cases |
1962-1969 |
4.5 |
14 |
4 |
1107 |
1970-1979 |
2.46 |
40 |
22 |
3519 |
1980-1989 |
1.54 |
52 |
36 |
3578 |
1990-2000 |
0.99 |
67 |
64 |
4325 |
*2000-2008 |
0.71 |
48 |
59 |
3999 |
Data for fallout is available from the UK annual reports of the Letcombe Research laboratory, from measurements made by the Atomic Energy Research Establishment Harwell and from the 2000 and earlier reports of the United Nations [16-18]. In table 2 the mean fallout doses (as calculated by the UK National Radiological Protection Board [19]) are given together with the Relative Risks obtained for the same period by the Bunch et al 2014 study.
Table 2: Annual estimated doses from atmospheric test fallout for England and Wales for the period identified by Bunch et al 2014 and Relative Risks of childhood leukemia 0-200m from high voltage power lines.
Period |
Annual mean fallout dose (mSv) [15] |
Relative Risk child leukemia 0-200m |
1962-1969 |
68 |
4.5 |
1970-1979 |
19 |
2.46 |
1980-1989 |
11 |
1.54 |
1990-1999 |
4.5 |
0.99 |
2000-2008 |
3 |
0.71 |
c2 = 7.62; p = 0.006 for linear trend in proportions.
The association between mean fallout dose and Relative risk was tested using the extended Mantel Haenszel c2 for trend. Result showed a highly significant association c2 = 7.62; p = 0.006.
The trend in between childhood leukemia risk and fallout doses is shown in figure 1.
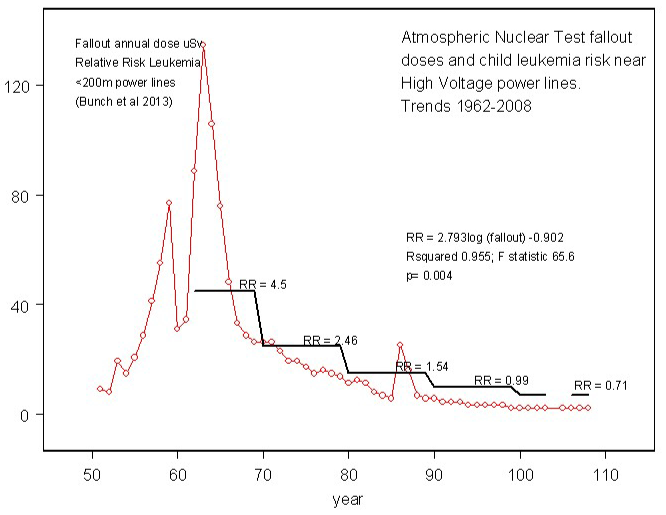
Figure 1: Trend in annual estimated doses from atmospheric test fallout for England and Wales (red) and Relative Risk of childhood leukemia within 200m of high voltage power lines in England Wales and Scotland. Year 100 = 2000. Note Chernobyl 1986 The best fit of the dose response was a logarithmic fit. RR = 2.79 log(dose) – 0.902. R2 = 0.955; F-statistic 65.6; p = 0.004.
The dose response relationship seen in the association is shown in figure 2. It is concave downwards or hogs-back.
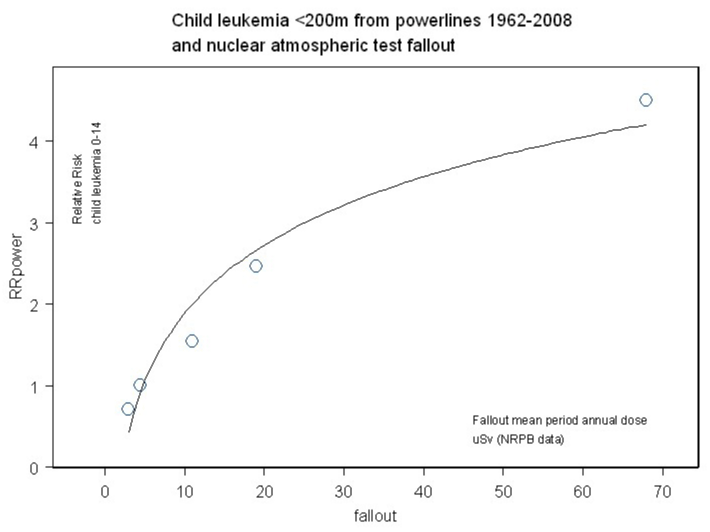
Figure 2: Relative Risk of child leukemia versus fallout doses. Statistically significant exponential relationship.
The conclusion of Bunch et al 2014 was that there was no physical process that had a variation over the period of their study which could explain the trend. They believed therefore that the earlier finding(s) had been a statistical artefact [3] and this was duly echoed in the media [20]. However, a possible cause of the association had been advanced by Henshaw’s group in 1999: they argued that the high voltages increased the concentration of fine and ultrafine particles in the vicinity of power lines through electrostatic effects involving the generation of corona ions [15]. Atmospheric tests caused global distribution of fallout, which included micron and sub-micron Uranium and Plutonium particles from the weapons themselves together with similar radioactive particles generated through condensation of the fireballs. The longer lived of these included the now well-known Caesium-137 and Strontium-90 radionuclides with physical half-lives of about 30 years.
The causal association between childhood leukemia and radioactive contamination has been a source of argument since the discovery of a 9-fold child leukemia cluster at the village of Seascale on the coast of the Irish Sea close to the nuclear fuel reprocessing plant at Sellafield. The resulting government enquiry exonerated radioactivity as a cause because it was argued by the UK National Radiological Protection Board that the doses to the parents and children were far too low [21]. The Chair of the Enquiry, Sir Douglas Black, was clearly puzzled by this and suggested that an independent scientific committee be formed to examine the scientific background and report back. This became the Committee on Medical Aspects of Radiation in the Environment (COMARE) a group which is still in existence today, funded by the Department of Health.
Between 1984, and the present day, COMARE have published 17 reports, most of these pertaining to the childhood leukemia clusters near nuclear sites. After Sellafield, increased childhood leukemia risk was found at nearly all the nuclear sites where studies were carried out [7-10] and a very large study from Germany also confirmed a statistically significant excess risk [8]. The latest COMARE 17th report continues to dismiss the possibility that the radiation exposures can be the cause of the leukemias, and for the same reason, the doses are too low [10].
If we unpack this assertion, there are two components which are open to question. The main one has to do with the concept of dose itself, as applied to the kinds of internal particulate exposures which must have been the only conceivable source of genetic damage in the power line children, and certainly one conceivable source of exposure in the Sellafield and nuclear site children and their parents. As has been argued elsewhere, the concept of dose cannot safely be applied to internal exposures, especially to radioisotopes with affinity for DNA or for particulates [22-24]. The transfer of radioactive contamination from intertidal sediment of the Irish Sea has been shown to occur and to contaminate the air within 2km of the coast, so the inhalation of radioactive micro- and nano-particles from this Sea-to-Land transfer is a possible vector. An increase in child leukemia was also found in a 1990 study of estuaries which are contaminated with radioactive particles, arguably by a similar cause [25]. Second, the relation between child leukemia and ionising radiation has not been established; and so the statement by NRPB and later COMARE about the dose being too low is not based on any real epidemiology except external exposure epidemiology like the ankylosing spondylitis studies [26] and the Japanese A-Bomb studies which have significant epidemiological problems [27].
There is another relevant concern. If child leukemia can be seen as a genetic anomaly or as a genetic damage in utero [28] then it is clear that increasing the exposure cannot linearly increase the effect, because at some level of exposure, the foetus will fail. This issue of the dose response for genetic and teratogenic effects was raised in the 1980s [29]. It may, in fact also be that the infant will die from some infection, since a precursor condition to the clinical expression of child leukemia will be an increased risk of infection [13]. Alternatively, the dose response will conform to the biphasic pattern due to death of the individual before birth. We should therefore expect a biphasic dose response, with a reduction in response at some point due to death of the individual before birth. The saturation seen in the dose response in Fig 2 can be explained by such a mechanism. The peak fallout years 1959-63 did not show a high level of childhood leukemia which has been argued by some [11,12] to show that low level internal exposures like those near the nuclear sites cannot cause any increase. However, there was a very significant increase in child leukemia recorded in Denmark where there was a continuous cancer registry operating over the early period of the testing [30,31]. The studies of the peak fallout periods [11,12] began at the top of the fallout peak and missed this increase. At the same time, there was a significant increase in infant mortality which correlated with the fallout peak [32,33]. Thus it is arguable that the lack of a major child leukemia effect at the height of the fallout was a result of infant mortality as a confounder.
For various reasons a plausible radionuclide contender here for the key causal exposure is particulate Uranium. First, the nuclear site clusters. Following the Black enquiry on Seascale a letter was sent by a Sellafield researcher drawing attention to large amounts of Uranium Oxide contamination from the early operation of the Windscale reactor [34]. Uranium oxide particles are long lived in the environment, are respirable and can be resuspended in the air [35]. Uranium particulates are released routinely to the atmosphere from nuclear reactor stacks: quantities are tabulated in UNSCEAR 2000 [12] and therefore there will be an excess air concentration near nuclear sites. This is also the case at the Atomic Weapons establishment Aldermaston where such particles are routinely measured in filters. There was a childhood leukemia cluster found in the area in 1998 [36]. In fact the exposures at Aldermaston are only to Uranium and Plutonium particles, not to the other fallout residue radionuclides. Another piece of evidence is the high level of leukemia found in Fallujah Iraq where the radioactivity exposures were only to Uranium particles [37,38]. The question of the radiobiological effects of Uranium has been discussed recently [22,39]. Uranium binds chemically to DNA and shows a wide range of anomalous genotoxic effects: the issue will not be discussed further here.
There is one further question of interest with regard to the magnitude of the dose. If childhood leukemia is a consequence of genetic damage prior to birth, then it may be seen as a congenital anomaly or a consequence of a congenital anomaly or anomalies. A recent review of congenital effects of radiation, largely based on effects reported by many different groups after Chernobyl, found that doses below 1mSv, as conventionally assessed, caused significant increases in a wide range of congenital conditions [40]. The same review pointed out that the current risk factor for human genetic effects is taken from mice, because no human congenital effects were seen in the A-Bomb Lifespan Study groups. It argued that this was because there were serious problems with the comparison groups in these A-Bomb studies since all had been equivalently exposed to uranium particles from the bombs, the so called black rain. Thus the LSS studies were of no value in establishing risk coefficients for internal exposures, particularly to Uranium particles. Consequently it is necessary to fall back on studies of those exposed to such contamination from Chernobyl. These show that the childhood leukemia effects at low dose are quite plausible. And they also show that at the higher doses, the observed effects disappear because the children die before birth.
There is a statistically significant association between the risk of child leukemia within 200m of a high voltage power line in England, Wales and Scotland, and the doses from atmospheric test fallout over the period 1962-2008. A possible explanation is inhalation exposure to radioactive particulates attracted to high voltage power lines though well-described mechanisms. Such a hypothesis is also capable of explaining child leukemia clusters near nuclear sites.
- Wertheimer E and Leeper N (1979) Electrical wiring configurations and childhood cancer. Am J Epidemiol 109: 273-284. [Crossref]
- Draper G, Vincent T, Kroll ME, Swanson J (2005) Childhood cancer in relation to distance from high voltage power lines in England and Wales: a case control study. BMJ 330: 1290. [Crossref]
- Bunch KJ, Keegan TJ, Swanson J, Vincent TJ and Murphy MFG (2014) Residential distance at birth from overhead high voltage power lines: childhood cancer in Britain 1962-2008. Br J Cancer 110: 1402-1408. [Crossref]
- Bithell JF, Dutton SJ, Draper GJ, Neary NM (1994) Distribution of childhood leukaemias and non-Hodgkin’s lymphomas near nuclear installations in England and Wales. BMJ 309: 501–505. [Crossref]
- Black RJ, Sharp L, Harkness EF, McKinney PA (1994) Leukaemia and non-Hodgkin’s lymphoma: incidence in children and young adults resident in the Dounreay area of Caithness, Scotland in 1968–91. J Epidemiol Community Health 48: 232–236. [Crossref]
- COMARE (1996) Fourth report. The incidence of cancer and leukaemia in young people in the vicinity of the Sellafield site, West Cumbria: further studies and an update of the situation since the publication of the report of the Black Advisory Group in 1984. HMSO, London.
- Gardner MJ, Snee MP, Hall AJ, Powell CA, Downes S, et al. (1990b) Results of case–control study of leukaemia and lymphoma among young people near Sellafield nuclear plant in West Cumbria. BMJ 300: 423-429. [Crossref]
- Kaatsch P, Spix C, Jung I, Blettner M (2008) Childhood leukemia in the vicinity of nuclear power plants in Germany. Dtsch Arztebl Int 105: 725-732. [Crossref]
- Sermage-Faure C, Laurier D, Goujon-Bellec S, Chartier M, Guyot-Goubin A, et al. (2012) Childhood leukemia around French nuclear power plants--the Geocap study, 2002-2007. Int J Cancer 131: E769-80. [Crossref]
- COMARE (2016) Seventeenth Report. Further considerations of the incidence of cancers around the nuclear installations at Sellafield and Dounreay. London: HMSO.
- Darby SC, Olsen JH, Doll R, Thakrar B, Brown PD, et al. (1992) Trends in childhood leukaemia in the Nordic countries in relation to fallout from atmospheric nuclear weapons testing. BMJ 304: 1005–1009. [Crossref]
- Wakeford R, Darby S C and Murphy M F (2010) Temporal trends in childhood leukaemia incidence following exposure to radioactive fallout from atmospheric nuclear weapons testing. Radiat Environ Biophys 49: 213–227. [Crossref]
- Chandran R, Hakki M, Spurgeon S (2012) Infections in leukemia. In: Azvedo S Sepsis—an ongoing challenge. IntechOpen. [https://www.intechopen.com/books/references/sepsis-an-ongoing-and-significant-challenge/infections-in-leukemia]
- Haynes R, Bentham G (1995) ‘Childhood leukemia in Great Britain and fallout from nuclear weapons testing.’ Journal of Radiological Protection 1: 37-43.
- Fews AP, Henshaw D, Wilding RJ, Keitch PA (1999) Corona ions from powerlines and increased exposure to pollutant aerosols. Int J Radiation Biol 75: 1523-1531. [Crossref]
- UNSCEAR 2000 (2000) The 2000 report of the United Nations Scientific Committee on the Effects of Atomic Radiation. Report to the General Assembly. Part 1. Sources. New York: United Nations.
- Playford K, Lewis GNJ, Carpenter RC (1992) Harwell: Radioactive Fallout in the Air and the Rain: Results to the end of 1990. Report AEA-EE-0362. Oxford UK: AEA Technology
- Agricultural Research Laboratory Letcombe Reports. Radioactivity in the Environment. Annual Reports of the Agricultural Research Laboratory, Letcombe. London: HMSO
- CERRIE (2004) The Report of the Committee Examining Radiation Risks from Internal Emitters. Chilton: NRPB.
- 20. Medical News Today (2014) "No childhood leukemia risk from power lines - Children who spend their early years living near overhead power lines are not at greater risk of developing childhood leukemia, according to researchers at the University of Oxford in the UK, who report their findings in the British Journal of Cancer." [http://www.medicalnewstoday.com/articles/272330.php]
- Black D (1984) Investigation of the possible increased incidence of cancer in West Cumbria. Report of the Independent Advisory Group.
- London: HMSOBusby C (2013) Aspects of DNA Damage from Internal Radionuclides, New Research Directions in DNA Repair, Prof. Clark Chen (Ed.), ISBN: 978-953-51-1114-6, InTech, DOI: 10.5772/53942. Available from: [http://www.intechopen.com/books/new-research-directions-in-dna-repair/aspects-of-dna-damage-from-internal-radionuclides]
- Busby C (2017) Child health and ionizing radiation: Science, Politics and European Law. Pediatric Dimensions 2: 1-4. [doi:10.15761/PD.1000150]
- Busby C (2017) Radiochemical Genotoxicity Risk and Absorbed Dose. Res Rep Toxi 1: 1.
- Alexander FE, Cartwright RA, McKinney PA, Ricketts TJ (1990) ‘Leukemia incidence, Social Class and Estuaries: an Ecological Ananlysis’ J Public Health Med 12: 109-117.[Crossref]
- Brown WM, Doll R (1965) Mortality from cancer and other causes after radiotherapy for ankylosing spondylitis. Br Med J 2: 1327-32. [Crossref]
- Busby C (2016) Letter to the Editor on &ldqu2021 Copyright OAT. All rights reservdies. Discrepancies between results and general perception.” By Bernard R Jordan. Genetics 204: 1627-1629.
- Greaves MF (1988) Speculations on the cause of childhood acute lymphoblastic leukemia. Leukemia 2: 120-125. [Crossref]
- Selevan SG, Lemasters GK (1987) The dose-response fallacy in human reproductive studies of toxic exposures. J Occup Med 29: 451-454. [Crossref]
- Clemmesen IH. Danish Cancer Registry registry data 1943- 1970. Complete set of data donated to author by Dr Clemmesen before his death.
- Busby C (2006) Wolves of Water. A Study Constructed from Atomic Radiation, Morality, Epidemiology, Science, Bias, Philosophy and Death. Aberystwyth: Green Audit.
- Whyte RK (1992) `First Day Neonatal Mortality since 1935: A Re-examination of the Cross Hypothesis’. BMJ 304: 343-346. [Crossref]
- Sternglass EJ (1971) ‘Environmental Radiation and Human Health,’ in Proceedings of the Sixth Berkeley Symposium on Mathematical Statistics and Probability. Neyman J (Edtr) (Berkeley, Calif.: University Press)
- Jakeman D (1984) Notes on the levels of radioactive contaminants in the Sellafield Area arising from discharges in the early 1950s. London: HMSO.
- Royal Society (2002) The health hazards of Depleted Uranium Weapons Part II. London: The Royal Society.
- Watson EA, Beral V, Buckle S, Bull D, Ryder H, et al. (1993) ‘Case control study of leukemia and non-Hodgkin lymphoma among children aged 0-4 years in West Berkshire and North Hampshire Health Districts’. BMJ 306: 615-621.[Crossref]
- Alaani S, Tafash M, Busby C, Hamdan, M, Blaurock-Busch E (2011) Uranium and other contaminants in hair from the parents of children with congenital anomalies in Fallujah, Iraq. Conflict and Health 5: 1-15.
- Busby C, Hamdan M, Ariabi E (2010) Cancer, infant mortality and birth sex-ratio in Fallujah, Iraq 2005-2009. Int J Environ Res Public Health 7: 2828-2837. [Crossref]
- Busby Christopher (2015) Editorial: Uranium Epidemiology. JJ Epidemiol Prevent 1: 009. [http://jacobspublishers.com/images/Epidemiology/J_J_Epidemiol_Prevent_1_2_009.pdf]
- Schmitz-Feuerhake I, Busby C, Pflugbeil S (2016) Genetic Radiation Risks-a neglected topic in the Low Dose debate. Environ Health Toxicol 31: e2016001. [Crossref]


