Abstract
Objective: This study was performed to determine whether renal function and graft origin affect serum levels of tumor necrosis factor-a (TNF-a), serum amyloid A (SAA) and C-reactive protein (CRP), among individuals having undergone renal transplantation (Tx).
Methods: In a sample of fifty kidney recipients, serum creatinine (Cr), SAA, TNF-α and CRP were quantitatively determined at several time-points, pre- and post-Tx (2nd, 6th, 14th day, 3 months post-Tx). Patients were grouped as follows: those presenting restored renal function (<1.5mg/dl three months post-Tx) versus those with mild renal dysfunction (>1.5mg/dl three months post-Tx), patients having received allograft from living donors versus patients having received cadaveric grafts.
Results: A total of 32 participants received grafts from living donor while 18 received cadaveric transplants. Two evenly numbered groups (N=25) were formed according to the presence of mild renal dysfunction. The differences of SAA values between those with and without restored renal function, as well as between living versus cadaveric graft recipients, were not significant at any time point. Similarly, no disparities were detected in TNF-a levels, between patients with serum Cr<1.5mg/dl and those with Cr>1.5mg/dl, or between recipients of living and cadaveric donors. Finally, serum CRP levels were homogeneous in individuals with and without mild renal dysfunction, as well as in patients receiving grafts from living and cadaveric donors throughout the three-month follow-up. In an exploratory analysis, serum CRP and cystatin-c were not correlated, at any of the time-points.
Conclusions: SAA, TNF-α and CRP are not differentiated by renal function and graft origin after renal transplantation.
Keywords
serum amyloid A, Tumor Necrosis Factor-α, C-reactive protein, cystatin – c, renal transplantation
Abbreviations
TNF-a-tumors necrosis factor -a; SAA-serum amyloid A; CRP-C-reactive protein; Tx-transplantation; Cr-creatinine; ESRD-end-stage renal disease; HD-hemodialysis; NK-natural killer cells; IL-6-interleukin 6; PD-peritoneal dialysis
Introduction
Kidney transplantation is the preferred medium of renal replacement therapy in patients with end-stage renal disease (ESRD). It offers better quality of life and longevity to patients [1]. Nevertheless, renal transplantation harbors a risk of graft loss. Acute graft rejection remains the major determinant of chronic rejection, which is the number one cause of graft loss and induces severe cardiovascular morbidity and mortality [2-4].
Kidney disease progression and allograft loss are associated with inflammatory processes. Published studies have already investigated inflammatory markers in the course of renal transplantation and have captured a strong relationship between inflammatory markers (pre- and post-transplantation) and graft outcome [5-7]. In greater detail, high plasma (as well as urinary) concentrations of tumor necrosis factor – α (TNF-a, a pro-inflammatory cytokine) have been related to higher risk of graft rejection and impaired graft function, through activation of T lymphocytes [8-11]. Elevated serum amyloid A (SAA, an acute phase protein) has also exhibited an association with kidney allograft rejection [12-17], while increased C-reactive protein (CRP, an acute phase protein, as well) is also considered a harbinger of chronic allograft dysfunction, graft rejection and cardiovascular mortality [6,7,17-19].
Considering the relationship of the aforementioned inflammatory biomarkers with post-transplantation outcomes, we decided to undertake the current study in order to investigate factors that could influence the serum level of TNF-a, SAA and CRP. Plasma levels of TNF-a, SAA and CRP were recorded in kidney transplant recipients pre- and throughout a three-month post-transplantation follow-up. Serum levels were compared between patients that received grafts from living donors and those who received cadaveric grafts. Moreover, plasma levels were compared between patients with and without restored renal function 3-months post-transplantation.
Patient and Methods
In this study, 50 patients (32 males, 18 females) of the kidney transplant unit of “Laiko” General Hospital of Athens, who received renal allografts were included. This study lasted from 27/03/2003 to 16/07/2004. The recipients belonged to the age range 15 - 70 (mean age = 42.5 years). Thirty-two patients received allografts from living donors and eighteen patients received cadaveric grafts.
All patients were treated with standard immunosuppressive therapy (Figure 1). A rejection episode was diagnosed in 10 of the patients and the anti-rejective therapy was effective. Rejection was identified by the increase of serum creatinine and was confirmed by allograft biopsy. All patients were followed for three months after transplantation. At this point, the average serum creatinine was 1,58 ± 0,4 mg/dl, while pre-transplantation levels were 7,41 ± 1,4. Twenty-five patients had Cr ≤ 1,5mg/dl (0,9 to 1,5, mean 1,3) and an even number of participants had Cr> 1,5mg/dl (1,5 to 2,7, mean 1,9).
Figure 1. Frequency of Patients per Standard Immunosuppressive Therapy
Fasting venous blood samples were collected in stable time points (during last week before transplantation, 2nd day, 6th day, 14th day and 90th day after transplantation). Blood samples were centrifuged, and serum samples were stored at -80o C. These conditions are appropriate and allow laboratory analysis after long-term storage of the samples. The changes of SAA, TNF-a, CRP and cystatin-c serum levels were assessed in all patients and were compared between patients with Cr ≤ 1.5mg/dl and patients with Cr> 1.5mg three months after transplantation, as well as between recipients of living and cadaveric grafts.
Statistical analysis
Analysis was performed using the statistical package SPSS® version 17 for Windows. Normality hypothesis for scale variables was tested by the Kolmogorov-Smirnov test. Differences in Pre-transplantation and the Post-transplantation time points (Day 2, Day 6, Day 14, Month 3rd) were evaluated using independent samples t-test (if normality assumption were met) or Mann-Whitney U test (if normality assumption were violated). The conventional threshold of α= 0.05 was set for the revelation of statistical significance. Finally, an exploratory analysis was performed. Cystatin C values were correlated to CRP concentrations via Pearson’s test, using the same statistical cut-off for significance.
Results
The changes of SAA are summarized per study group in Table 1. SAA showed an early increase post-Tx with a peak of its value on the 6th day and subsequent decrease three months post-transplantation. Figure 2 illustrates serum SAA trajectories in the whole sample, as well as per study group. No significant difference was established between participants with and without mild renal impairment, throughout the three-month follow-up. Similarly, serum SAA recorded comparable patterns in the course of the three-month follow-up, irrespective of the origin of the allograft, i.e., living vs. cadaveric donor.
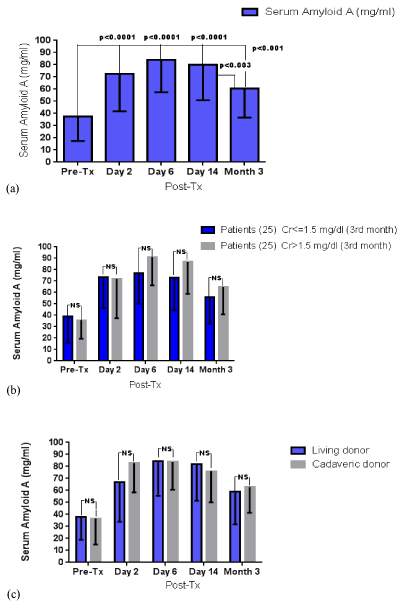
Figure 2. SAA levels throughout the three-month follow-up (a) in the whole sample, (b) according to the presence of mild renal impairment and (c) according to the graft origin; NS: non-significant.
Table 1. Mean serum amyloid–A values (± standard deviations) in the course of the three-month follow-up. P values correspond to post- (post-Tx) vs. pre-transplantation (pre-Tx) differences. SAA was measured in μg/ml
All patients (N=50) |
Pre-Tx |
Post-Tx |
2nd day |
6th day |
14th day |
90th day |
37,31 ± 19,97 |
72,74 ± 30,91 P<0,0001 |
84,09 ± 26,60 P<0,0001 |
79,90 ± 28,98 P<0,0001 |
60,36 ± 23,77 P<0,001 |
Cr≤ 1,5mg/dl (N=25) |
38,93 ± 23,27 |
73,60 ± 27,57 P<0,0001 |
76,94 ± 26,87 P<0,0001 |
72,98 ± 28,82 P<0,0001 |
55,94 ± 23,20 P<0,001 |
Cr> 1,5mg/dl (N=25) |
35,69 ± 16,35 |
71,87 ± 34,47 P<0,0001 |
91,24 ± 24,93 P<0,0001 |
86,81 ± 28,00 P<0,001 |
64,77 ± 23,98 P<0,0001 |
Living donor (N=32) |
38,01 ± 19,17 |
66,91 ± 32,94 P<0,0001 |
84,28 ± 28,74 P<0,0001 |
82,01 ± 30,65 P<0,0001 |
58,95 ± 27,17 P<0,001 |
Cadaveric donor (N=18) |
36,71 ± 21,83 |
82,98 ± 24,51 P<0,0001 |
83,76 ± 23,10 P<0,0001 |
76,15 ± 26,14 P<0,0001 |
62,86 ± 21,52 P<0,001 |
Cr: creatinine; N=number of participants
The changes of serum TNF-a are summarized per study group in in Table 2. TNF-a recorded a progressive decrease throughout the three-month post-transplantation monitoring. Figure 3 demonstrates serum TNF-a trajectories in the whole sample, as well as per study group. Much alike SAA, there were no disparities between those with and without mild renal impairment, as well as between living vs. cadaveric graft recipients.
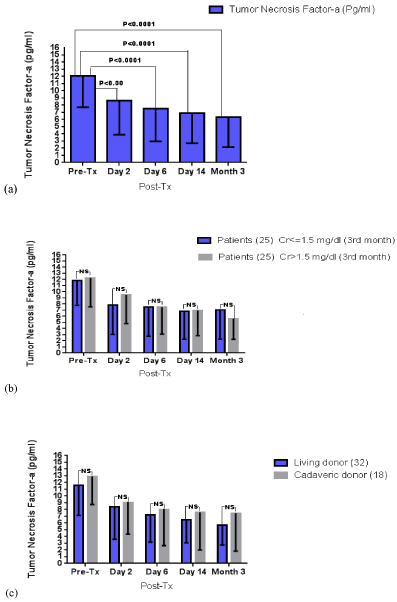
Figure 3. Serum TNF-a levels throughout the three-month follow-up (a) in the whole sample, (b) according to the presence of mild renal impairment and (c) according to the graft origin; NS: non-significant
Table 2. Mean TNF-a (Tumor Necrosis Factor -a) levels (± standard deviations) in the course of the three-month follow-up. P values correspond to post- (post-Tx) vs. pre-transplantation (pre-Tx) differences. TNF-a was measured in pg/ml
All patients (N=50) |
Pre-Tx |
Post-Tx |
2nd day |
6th day |
14th day |
90th day |
12,07 ± 4,36 |
8,65 ± 4,76 P<0,001 |
7,52 ± 4,56 P<0,0001 |
6,87 ± 4,15 P<0,0001 |
6,32 ± 4,15 P<0,0001 |
Cr ≤ 1,5mg/dl (N=25) |
11,86 ± 4,06 |
7,81 ± 4,75 P<0,001 |
7,53 ± 4,80 P<0,001 |
6,80 ± 4,56 P<0,0001 |
7,02 ± 4,76 P<0,0001 |
Cr> 1,5mg/dl (N=25) |
12,27 ± 4,72 |
9,49 ± 4,71 P<0,05 |
7,52 ± 4,42 P<0,001 |
6,95 ± 4,11 P<0,0001 |
5,62 ± 3,38 P<0,0001 |
Living donor (N=32) |
11,60 ± 4,47 |
8,37 ± 4,79 P<0,007 |
7,19 ± 4,02 P<0,0001 |
6,47 ± 341 P<0,0001 |
5,68 ± 2,94 P<0,0001 |
Cadaveric donor, (N=18) |
12,90 ± 4,15 |
9,14 ± 4,80 P<0,01 |
8,12 ± 5,47 P<0,006 |
7,58 ± 5,58 P<0,003 |
7,46 ± 5,63 P<0,002 |
Cr: creatinine; N=number of participants
Post-transplantation serum CRP in all patients of all subgroups, showed an early increase, presenting a peak on the 2nd day and after transplantation and a subsequent progressive decrease until the completion of the follow-up (Table 3). Similar to TNF-a and SAA, CRP values documented comparable trajectories in the course of the follow-up, without any between group differences (based on renal function and graft origin) at any time point (Figure 4).
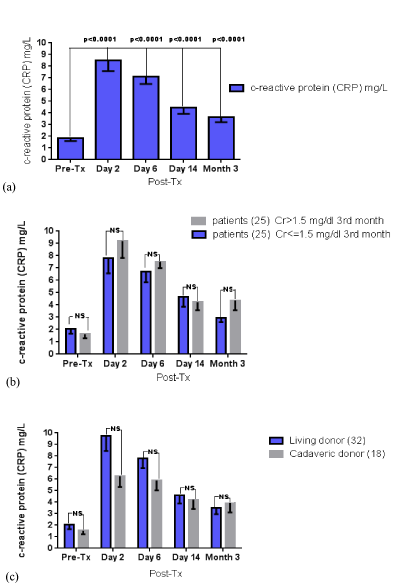
Figure 4. Serum CRP levels throughout the three-month follow-up (a) in the whole sample, (b) according to the presence of mild renal impairment and (c) according to the graft origin; NS: non-significant
Table 3. Mean CRP (C reactive protein) levels (± standard deviations) in the course of the three-month follow-up. P values correspond to post- (post-Tx) vs. pre-transplantation (pre-Tx) differences. CRP was measured in mg/dL
All patients (N=50) |
Pre-Tx |
Post-Tx |
2nd day |
6th day |
14th day |
90th day |
1.85 + 0.26 |
8.49 ± 0.91
P<0.0001 |
7.11 ± 0.63
P<0.0001 |
4.45 ± 0.52
P<0.001 |
3.66 ± 0.45
P=0.001 |
Cr≤ 1,5mg/dl (N=25) |
2.07 ± 0.40 |
7.78 ± 1.21
P<0.0001 |
6.70 ± 0.86
P<0.0001 |
4.67 ± 0.82
P<0.0001 |
2.98 ± 0.34
N.S |
Cr> 1,5mg/dl (N=25) |
1.63 ± 0.33 |
9.20 ±1.38
P<0.0001 |
7.51 ± 0.52
P<0.0001 |
4.23 ± 0.67
P<0.001 |
4.37 ± 0.81
P<0.001 |
Living donor (N=32) |
2.03 ± 0.37 |
9.47 ± 1.29
P<0.0001 |
7.80 ± 0.85
P<0.0001 |
4.59 ± 0.70
P<0.002 |
3.50 ± 0.54
P<0.03 |
Cadaveric donor, (N=18) |
1.53 ± 0.31 |
6.25 ± 0.93
P<0.0001 |
5.88 ± 0.85
P<0.0001 |
4.21 ± 0.79
P<0.003 |
3.93 ± 0.81
P<0.009 |
Cr: creatinine; N=number of participants; N.S: non significant
Finally, the correlation between serum CRP and cystatin-c was performed in the whole sample, at every time point (Figure 5). These indices were not correlated at any time point in the course of the three-month follow-up (Figure 5).
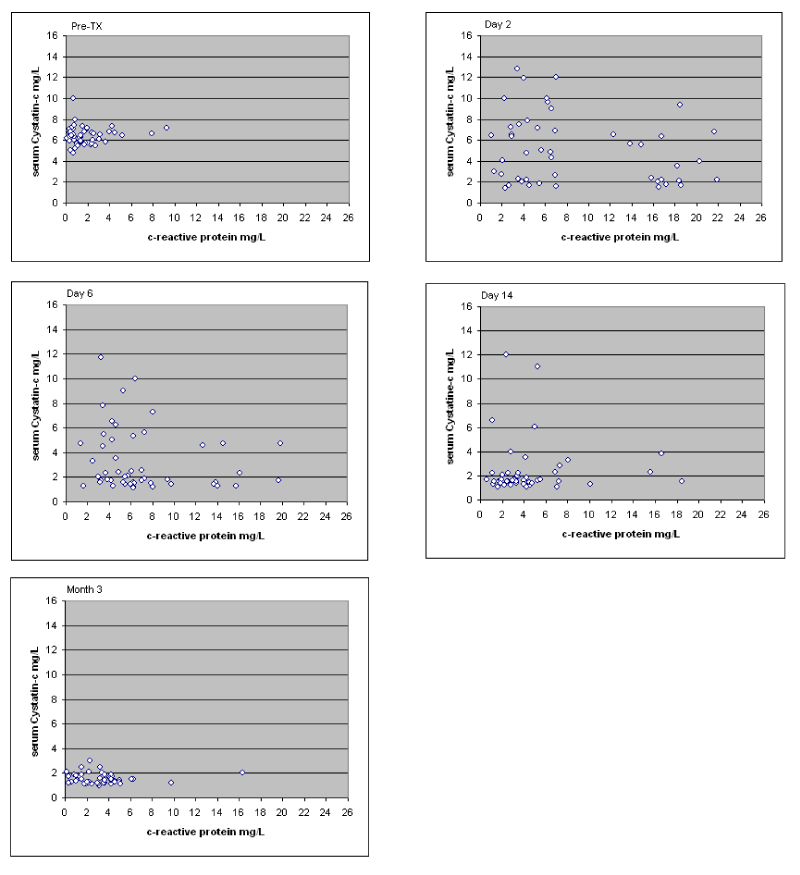
Figure 5. Correlations between c-reactive protein and cystatin-c throughout the three-month follow-up; Pearson’s r: pre-transplantation r=0.099, post- transplantation: 2nd day r=-0.143, 6th day r=-0.228, 14th day r=0.097, 3 months r=-0.121
Discussion
There is strong evidence that acute phase proteins and other inflammatory indices can provide useful information about the early recognition and monitoring of allograft malfunction. SAA, TNF-a and CRP are considered important such biomarkers. These biomarkers are determined in the post-transplantation course to evaluate the risk of allograft complications as well as effective management of the whole process [12,20,21]. In this study, it was established that serum levels of TNF-a, SAA and CRP are independent from two important transplantation-related parameters, renal function after transplantation and graft origin. This finding suggests that these biomarkers may be utilized in terms of risk stratification and prognostic value, independently of these pivotal parameters.
Serum Amyloid-A (SAA) is an acute phase protein with a molecular weight of 12000, which is synthesized and degraded in the liver, free of any renal metabolic processes. It is reported that SAA can increase days (one or more) before creatinine rise, foreboding acute graft rejection. Some studies suggest that the close monitoring of SAA concentrations in kidney transplant recipients could facilitate the early diagnosis of acute allograft rejection [12,16,20]. Of note, SAA responds earlier and more sensitively than CRP to inflammatory stimuli, even in patients on corticosteroids. Unfortunately, SAA responds sensitively to surgical trauma as well, and its rise is of low specificity complication-wise in the early post-transplantation period. However, during the postoperative period, the ratio of SAA and CRP values is about 6:1, while in allograft rejection this ratio ranges between 7-10:1; hence, the specificity and negative prognostic value of SAA may be elevated by assessing its relative increase, compared to CRP.
Tumor Necrosis Factor-a (TNF-a) is an endotoxin-induced glycoprotein, originally described as a circulating factor that can cause necrosis of tumor. However, accumulating evidence demonstrated that TNF-a is a key regulator of the inflammatory response and is implicated in the pathophysiological course of multiple disorders [14,15]. TNF-a, along with other pro-inflammatory cytokines (e.g., IL-6), are elevated in patients undergoing hemodialysis (HD). These indices are supposed to reflect the prominent inflammatory activity of HD patients and assume a role in their general cachectic state and ill-being. TNF-a concentrations are also elevated during kidney transplant rejection. In allograft rejection, activated immune cells involving macrophages, CD4+ T cells, natural killer (NK) cells, neutrophils, and endothelial cells have been shown to produce exaggerated amounts of TNF-a. Of note, combined serial assays of serum TNF-a and serum creatinine have greater predictive value than either test alone for the early diagnosis of acute rejection [2,14,15], while TNF-a gene polymorphisms may reveal a subgroup of graft recipients under an elevated risk of acute and delayed rejection [4]. Intriguingly, high TNF-a levels in donors may confer an additional risk for delayed graft rejection in kidney recipients.
Among the investigated indices, CRP constitutes the least specific marker of undergoing inflammatory processes. CRP, the prototypical acute phase reactant marker of inflammation is synthesized predominantly by hepatocytes under the predominant control of interleukin-6 (IL-6) and other inflammatory cytokines. The major determinant of CRP levels is the rate of synthesis by the liver and not its excretion through the kidney [22,23]. HD individuals and peritoneal dialysis (PD) patients show evidence of activated inflammatory responses with increased serum levels of non-specific markers of inflammation and pro-inflammatory cytokines. An increase of CRP levels is expected in 30-50% of patients undergoing HD and PD therapy. In the post-transplantation course, surgical trauma is considered a major cause of increased CRP during the first 4 days, while treatment toxicity, infections and other confounders limit its sensitivity later in the post-transplantation period. Tardive elevations of CRP are supposed to reflect primary immune responses towards the graft better and appear to be related to rejection activities [23-28]. Overall, there are contradictory findings for the association between acute rejection and high pre-transplantion CRP levels. Of note, high pre-transplantion CRP levels may be strong risk factor for rejection after transplantation [18,29].
The overall benefit of renal transplantation is limited by renal transplant loss or death, mostly due to cardiovascular disease. The common pathogenesis between allograft nephropathy and cardiovascular disease, includes inflammation and accelerated atherosclerosis. Hence biomarkers of inflammation and atherosclerosis may possess optimum properties for predictive purposes and be appropriate for risk stratification strategies. Future research ought to shed additional light into this matter, and contribute further to the prevention of transplantation-related complications.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
AG, participated to conception and design of study, collection and interpretation of data and contributed to writing the manuscript. DP, contributed to conception and design of study and was involved in acquisition, analysis and interpretation of data. TK and MR, were involved in the interpretation and analysis of data, in manuscript preparation and writing and made critical revision. ANK, was involved in analysis and interpretation of data. JB, contributed to design of study, acquisition and interpretation of data. AK, contributed to conception and design of study and made critical revision. All authors have approved the final manuscript and certify that this manuscript represents valid work and has not been previously published.
References
- Lima JR, Salgado JV, Ferreira TC, Oliveira MI, Santos AM, et al. (2011) Cystatin C and inflammatory markers in kidney transplant recipients. Rev Assoc Med Bras 57: 347-52. [Crossref]
- Cottone S, Palermo A, Vaccaro F, Mulè G, Guarneri M, et al. (2007) Inflammation and endothelial activation are linked to renal function in long-term kidney transplantation. Transpl Int 20: 82-87. [Crossref]
- Palermo A, Mulè G, Vadalà A, Vaccaro F, Guarneri M, et al. (2008) Relationship of transforming growth factor-beta(1) with tumour necrosis factor-alpha and endothelial activation in patients with stable renal transplantation. Nephrology (Carlton) 13: 164-170. [Crossref]
- Mandegary A, Azmandian J, Soleymani S, Pootari M, Habibzadeh SD, et al. (2013) Effect of donor tumor necrosis factor-alpha and interleukin-10 genotypes on delayed graft function and acute rejection in kidney transplantation. Iran J Kidney Dis 7: 135-141. [Crossref]
- Roshdy A, El-Khatib MM, Rizk MN, El-Shehaby AM (2012) CRP and acute renal rejection: a marker to the point. Int Urol Nephrol 44: 1251-1255. [Crossref]
- Fink JC, Onuigbo MA, Blahut SA, Christenson RH, Mann D, et al. (2002) Pretransplant serum C-reactive protein and the risk of chronic allograft nephropathy in renal transplant recipients: a pilot case-control study. Am J Kidney Dis 39: 1096-1101. [Crossref]
- Pegues MA, McCrory MA, Zarjou A, Szalai AJ (2013) C-reactive protein exacerbates renal ischemia-reperfusion injury. Am J Physiol Renal Physiol 304: F1358-65. [Crossref]
- Hoffmann U, Bergler T, Rihm M, Krüger B, Rümmele P, et al. (2009) Upregulation of TNF receptor type 2 in human and experimental renal allograft rejection. Am J Transplant 9: 675-686 [Crossref]
- Budak D, Yilmaz VT, Akbas H, Suleymanlar G, Yucel G (2015) Association between graft function and serum TNF-α, TNFR1 and TNFR2 levels in patients with kidney transplantation. Ren Fail 37: 871-876. [Crossref]
- Wiggins MC, Bracher M, Mall A, Hickman R, Robson SC, et al. (2000) Tumour necrosis factor levels during acute rejection and acute tubular necrosis in renal transplant recipients. Transpl Immunol 8: 211-215. [Crossref]
- Senturk Ciftci H, Demir E, Savran Karadeniz M, Tefik T, Yazici H, et al. (2018) Serum and Urinary Levels of Tumor Necrosis Factor-Alpha in Renal Transplant Patients. Exp Clin Transplant 16: 671-675. [Crossref]
- Fukuda Y, Hoshino S, Tanaka I, Yoneya T, Takeshita T, et al. (2000) Examination of serum amyloid A protein in kidney transplant patients. Transplant Proc 32: 1796-1798. [Crossref]
- Simmons EM, Langone A, Sezer MT, Vella JP, Recupero P, et al. (2005) Effect of renal transplantation on biomarkers of inflammation and oxidative stress in end-stage renal disease patients. Transplantation 79: 914-919. [Crossref]
- Sonkar GK, Usha, Singh RG (2009) Evaluation of serum tumor necrosis factor alpha and its correlation with histology in chronic kidney disease, stable renal transplant and rejection cases. Saudi J Kidney Dis Transpl 20: 1000-1004. [Crossref]
- El-Gezawy EM, Eldin EN, Mohamed WS, Saied SA, et al. (2013) Tumor necrosis factor-alfa and monocyte chemoattractant protein-1 gene polymorphisms in kidney transplant recipients. Saudi J Kidney Dis Transpl 24: 688-695. [Crossref]
- Hartmann A, Eide TC, Fauchald P, Bentdal O, Herbert J, et al. (1997) Serum amyloid A protein is a clinically useful indicator of acute renal allograft rejection. Nephrol Dial Transplant 12: 161-166. [Crossref]
- Fukuda Y, Kanbe M, Sumimoto R, Yoneya T, Takeshita T, et al. (1998) Examination of serum amyloid A protein in kidney transplant patients-comparison of serum amyloid A and C-reactive protein for monitoring the occurrence of renal-allograft-related complications. Hiroshima J Med Sci 47: 63-67. [Crossref]
- Krüger B, Walberer A, Debler J, Böger CA, Farkas S, et al. (2001) Is inflammation prior to renal transplantation predictive for cardiovascular and renal outcomes? Atherosclerosis 210: 637-642.
- Gurlek Demirci B, Sezer S, Colak T, Sayin CB, Tutal E, et al. (2015) Post-transplant C-reactive protein predicts arterial stiffness and graft function in renal transplant recipients. Transplant Proc 47: 1174-1177. [Crossref]
- Casl MT, Bulatovic G, Orlić P, Sabljar-Matovinović M (1995) The diagnostic capacity of serum amyloid A protein for early recognition of kidney allograft rejection. Nephrol Dial Transplant 10: 1901-1904. [Crossref]
- Speeckaert MM, Speeckaert R, Laute M, Vanholder R, Delanghe JR (2012) Tumor necrosis factor receptors: biology and therapeutic potential in kidney diseases. Am J Nephrol 36: 261-270. [Crossref]
- van Ree RM, de Vries AP, Oterdoom LH, The TH, Gansevoort RT, et al. (2005) Abdominal obesity and smoking are important determinants of C-reactive protein in renal transplant recipients. Nephrol Dial Transplant 20: 2524-2531. [Crossref]
- van Ree RM, Oterdoom LH, de Vries AP, Gansevoort RT, van der Heide JJ, et al. (2007) Elevated levels of C-reactive protein independently predict accelerated deterioration of graft function in renal transplant recipients. Nephrol Dial Transplant 22: 246-253. [Crossref]
- Fink JC, Onuigbo MA, Blahut SA, Christenson RH, Mann D, et al. (2002) Pretransplant serum C-reactive protein and the risk of chronic allograft nephropathy in renal transplant recipients: a pilot case-control study. Am J Kidney Dis 39: 1096-1101. [Crossref]
- Oyen O, Wergeland R, Bentdal O, Hartmann A, Brekke IB, et al. (2001) Serial ultrasensitive CRP measurements may be useful in rejection diagnosis after kidney transplantation. Transplant Proc 33: 2481-2483. [Crossref]
- Their M, Rönnholm K, Sairanen H, Holmberg C, Jalanko H (2002) Serum C-reactive protein in pediatric kidney and liver transplant patients. Pediatr Transplant 6: 153-60. [Crossref]
- Sezer S, Akcay A, Ozdemir FN, Kulah E, Arat Z, et al. (2004) Post-transplant C-reactive protein monitoring can predict chronic allograft nephropathy. Clin Transplant 18: 722-725. [Crossref]
- Ozdemir NF, Elsurer R, Ibis A, Arat Z, Haberal M (2007) Serum C-reactive protein surge in renal transplant recipients: link with allograft survival. Transplant Proc 39: 934-937. [Crossref]
- Varagunam M, Finney H, Trevitt R, Sharples E, McCloskey DJ, et al. (2004) Pretransplantation levels of C-reactive protein predict all-cause and cardiovascular mortality, but not graft outcome, in kidney transplant recipients. Am J Kidney Dis 43: 502-507. [Crossref]





