Fifty patients with severe penetrating eye injury (PEI) and twenty-two nearly healthy men-volunteers were examined. Despite the locality of injury, early traumatic period prior to conservative therapy of PEI patients demonstrated systemic changes in their immune system. Proliferative response of lymphocytes in cultures with phytohemagglutinin-P (PHA) was considerably reduced; lymphocyte sensitivity to suppressing action of beta-adrenoreceptors was elevated in vitro. The intensity in PHA-induced proliferative response of lymphocytes and its sensitivity to suppressive action of beta-adrenoreceptor agonist (hexoprenaline sulfate, 10-6 M) were depended from cortisol level during early traumatic period.. PHA-induced blast-transformation of lymphocytes was found to be elevated during the late traumatic period under the conventional therapy including glucocorticoids. The inclusion of myelopidum into therapy resulted in extending the suppression of PHA-induced proliferative response of lymphocytes, and did not abolish the decrease in interferon-gamma production in cell cultures, and enhanced the anti-inflammatory effect of conventional therapy. Proliferative response of lymphocytes with thymus-dependent polyclonal activator of B-lymphocytes pokeweed mitogen (PWM) during the early traumatic period was found to be reduced in 72 h-cultures, and be elevated in 96 h-cultures. In addition, PWM-induced proliferative response of lymphocytes was elevated both in 72 h- and 96 h-cultures in the late traumatic period, and most markedly with myelopidum inclusion in therapy. The increase in detection frequency of antibodies to eye tissue antigens (cornea specific protein BCP 54, alpha-crystallin and S-antigen) was not revealed.
penetrating eye injury, immune system, immunomodulation, myelopidum, myelopeptides, stress, cortisol, beta-adrenergic receptors
Abbreviations:
ACAID: Anterior Chamber Associated Immune Deviation; BCP 54: Bovine Corneal Epithelial 54-kD Protein; DTH: Delayed-Type Hypersensitivity; ELISA: Enzyme-Linked Immunosorbent Assay; IFN-: Interferon-Gamma; Ig: Immunoglobulin; IL: Interleukin; PEI: Penetrating Eye Injury; PHA: Phytohemagglutinin-P; PWM: Pokeweed Mitogen
Penetrating eye injury (PEI) is an example of spatially limited injury entailing not only local responses, including the impairment of immunosuppression mechanisms in this immunologically privileged organ itself, but also systemic stress-related changes in the immune system in response to the hazard of the loss of an organ important for receiving information [1-3]. The mechanisms of these changes in PEI have not been studied sufficiently; most researchers consider the anterior chamber associated immune deviation (ACAID) to be the leading phenomenon [4-6]. The main aim of penetrating eye injury therapy is directed regulation of traumatic inflammation with minimization of secondary damage events and optimization of regeneration [1,7]. One of approaches is the inclusion of immunomodulators in complex therapy, particularly myelopidum (a highly purified mixture of myelopeptides with molecular mass of 500-3000 Da being developed in Russia by R.V. Petrov, A.A. Mikhailova and coauthors [8,9]). In experimental model of penetrating eye injury the myelopidum inclusion in complex therapy results in reduction of inflammatory cell infiltration in a scar and perifocal tissues, promotes the formation of mature fibrous structures within a scar, favors the epithelization of corneal damaged area in the early traumatic period, abolishes the suppression of antibody formation under the immune response to thymus-dependent antigen in trauma and the introduction of conventional therapy preparations, but does not lower the degree of intensity in delayed-type hypersensitivity (DTH) reaction suppression [10].
The aim of work was to investigate an immune system changes, involvement of glucocorticoid and beta-adrenergic mechanisms therein, and to assess the effectiveness of myelopidum inclusion in complex therapy of patients with penetrating eye injury.
Patients
Clinical and immunological study was conducted in 50 male patients (19 to 49 years, average age - 35 years) with the severe penetrating eye injury in dynamics of traumatic process: first - on admission to hospital (on the 1-3rd day after injury before the start of conservative therapy), repeated - at hospital discharge (12 to 14 days of injury). All patients were treated at the ophthalmology department of the municipal health care institutions " F.H. Gral Perm City Clinical Hospital No. 2". All examinations and treatment were carried out in accordance with the requirements of the Ethics Committee of the hospital. Depending on the scheme of therapy all patients were divided into two groups: 1st (main group, 27 men) received a complex treatment - standard therapy in combination with myelopidum; 2nd (comparison group, 23 men) - standard therapy alone. On the day of admission to an ophthalmic hospital there was performed primary surgical treatment of wounds to the extent appropriate to each case using microsurgical techniques. Subsequently, all patients received standard therapy, including local and general application of antibacterial drugs, dexamethasone, diclofenac sodium, antihistamine drugs, enzymes, vitamins, antioxidants, osmotic drugs. Dexamethasone was administered to all those affected locally in the instillation and subconjunctival injections (2 mg/day for all period of hospitalization), and parenterally on the pulse therapy principle (8 mg/day intravenously for 3 days) to suppress the development of autoimmunity and to enhance anti-inflammatory effect of therapy. Myelopidum was used in a dose of 3 mg/day (1.5 mg subcutaneously in the mastoid on the side of the injured eye and intramuscularly 1 time per day every day for 5 days). Selection of patients in the main group and comparison group was conducted by random sampling. The 3rd (control) group included 22 healthy male volunteers (18 to 48 years, average age - 34 years).
Common ophthalmologic examination was performed for each patient. Evaluating the effectiveness of treatment was carried out according to the sign of clinical effect: 1 - ocular inflammatory process as expressed; 2 - the absence of positive dynamics of the objective manifestations of inflammation, a slight decrease in subjective discomfort; 3 - slight clinical improvement, presence of inflammation; 4 - significant clinical improvement, presence of residual symptoms of inflammation; 5 - recovery, the absence of symptoms of inflammation [1].
Cultures
Peripheral blood leukocytes were isolated by spontaneous sedimentation. Proliferative response of lymphocytes was studied in cultures with phytohemagglutinin-P (PHA, cat. no. L9132, Sigma, USA) in concentrations of 2.5, 5.0, 10.0 and 20.0 microgram/ml in 199 medium supplemented with 2 mM L-glutamine, 10 mM HEPES, 100 microgram/ml gentamicin sulfate and 10% autoplasma (2 × 105 cells per well of a round 96-well plate, the total volume of 0.2 ml of culture). After 48 h of cultivation in a humidified atmosphere at 37°C 1 microCi methyl-3H-thymidine ("Isotope", St.-Petersburg) was added to each well. After 72 h, cells were harvested on Millipore membrane filters with a pore diameter of 0.4 microns. The level of radioactivity was assessed by liquid scintillation counter "Guardian" WinSpectral DSA 1414-03 "Wallac" (Finland).
To estimate the sensitivity of the PHA-induced lymphocyte proliferative response in vitro to beta-adrenergic regulation, the beta-adrenoreceptor agonist hexoprenaline sulfate ("gynipralÒ", Nycomed, Austria) was added to some cultures at a concentration of 10–6 M.
Production of interferon-gamma (IFN-γ) was estimated in the similar cultures with 20 microgram/ml of PHA 48 h after by enzyme-linked immunosorbent assay (ELISA). Commercial test systems were used (²Protein contour" Ltd., St.-Petersburg, Russia). Analysis of culture supernatants was carried out in accordance with the manufacturer instructions. Optical density was recorded on an automatic spectrophotometer Sanofi Diagnostics Pasteur PR 2100 (France) at a wavelength of 450 nm with a cut-off filter 620 nm.
Proliferative response of lymphocytes with thymus-dependent B-cell mitogen - pokeweed mitogen (PWM, cat. no. L8777, Sigma) in concentrations of 0.625, 1.25, 2.5, 5.0 and 10.0 micrograms/ml was assessed in 72 - and 96-hour cultures using a method similar to cultures with PHA.
Antibodies to eye tissue antigens
The presence of antibodies to eye tissue antigens (the cornea specific protein BCP 54, alfa-crystallin and S-antigen) was determined in plasma of peripheral blood by ELISA.
To assess the effect of myelopidum on the frequency of antibody detection in the plasma of peripheral blood to the eye tissue antigens in patients with penetrating eye injury the presence of antibodies to corneal protein BCP 54, alfa-crystallin lens, retinal S-antigen of the retina was determined by ELISA using test systems produced by the Department of Experimental and Clinical Immunology, State health agencies "Russian Eye and Plastic Surgery of the Ministry of Health of the Russian Federation" [11-13]. The analysis was performed by the manufacturer's instructions using an analyzer MultiscanPlus (²Labsystems², Finland). In case of an excess optical density by the value of 0.1 or more, the ELISA results with the analyzed serum were considered as positive. Optical density in "negative" control (pool of serum of 100 healthy volunteers) was less than 0.1.
Сortisol concentration
The cortisol concentration in peripheral blood serum was determined using the competitive variant of ELISA performed by means of a test system from Xema Co., Ltd. (Moscow, Russia).
Statistical analysis
The results are presented in figures and text as mean ± standard errors (se). One-way ANOVA followed by Duncan's test for multiple comparison were employed to determine the significance of the differences between means. The significance of differences between two paired values was assessed by paired Student’s t-test. The U-Mann-Whitney test was used for the analysis of clinical efficacy.
Changes of lymphocyte proliferative response in cultures with PHA and the effect of beta-adrenergic agonist hexoprenaline sulfate in vitro under penetrating eye injury
Considering the key role of T lymphocytes in the progression of complications due to penetrating eye injury the evaluation of lymphocyte proliferative response in cultures with PHA was made in the course of traumatic process and treatment (Table 1). For the purpose of determination the involvement of mechanisms associated with beta-adrenoreceptors in traumatic immunomodulation the effect of beta-adrenoreceptor agonist hexoprenaline sulfate on PHA-induced proliferative response of lymphocytes was examined in vitro. The suppression of PHA-induced lymphocyte proliferative response in cultures without beta-adrenoreceptor agonist was established in the early traumatic period, the increase in its level was detected in patients with conventional therapy in the late traumatic period (Table 1). The treatment with myelopidum favored the retaining of lymphocyte proliferation suppression in cultures with suboptimal PHA concentration (2.5 microgram/ml) and without mitogen (p<0.05 relatively to patients receiving conventional therapy).
Table 1. Changes of lymphocyte proliferative response in cultures with PHA and the in vitro effect of beta-adrenergic agonist hexoprenaline sulfate (10-6 M) under penetrating eye injury.
Con-centra-tion
of PHA,
micro-gram/ml |
Cul- ture |
Control group (n=10) |
Primary examination
of patients (n=20) |
Re-examination of patients |
myelopidum+ standard therapy (n=10) |
standard therapy (n=10) |
0 |
Without agonist |
3.5508 ± 0.0734 (3554) |
3.3148 ± 0.0707 (2064) |
3.2958 ± 0.0917 (1976)* b |
3.5649 ± 0.0787 (3672) |
|
With agonist |
3.6470 ± 0.0668 (4436) |
3.2454 ± 0.0674 (1759) |
3.5037 ± 0.0864 (3189) |
3.6521 ± 0.1557 (4488) |
2.5 |
Without agonist |
3.8902 ± 0.1152 (7766) |
3.5370 ± 0.0808 (3444)* |
3.6218 ± 0.1129 (4186)b |
4.0817 ± 0.1344 (12069)а |
|
With agonist |
3.8611 ± 0.0796 (7263) |
3.3955 ± 0.0795 (2486)*# |
3.5398 ± 0.1400 (3466)* |
3.7424 ± 0.1135 (5526) а# |
5.0 |
Without agonist |
4.0792 ± 0.1228 (12002) |
3.6568 ± 0.1018 (4538)* |
3.8208 ± 0.1161 (6620) |
4.1770 ± 0.1162 (15031)а |
|
With agonist |
4.0691 ± 0.1162 (11723) |
3.5136 ± 0.1140 (3263)*# |
3.7776 ± 0.1209 (5993) |
3.8808 ± 0.1505 (7600)# |
10.0 |
Without agonist |
4.3980 ± 0.0695 (25003) |
3.7833 ± 0.0914 (6072)* |
4.1226 ± 0.0991 (13263)а |
4.2505 ± 0.1506 (17802)а |
|
With agonist |
4.2272 ± 0.0915 (16873)# |
3.6439 ± 0.1026 (4405)*# |
3.9809 ± 0.1298 (9570) |
4.0761 ± 0.1637 (11915) а |
20.0 |
Without agonist |
4.2991 ± 0.1000 (19919) |
4.0421 ± 0.0872 (11018) |
4.2962 ± 0.0941 (19781) |
4.5402 ± 0.1411 (34687)а |
|
With agonist |
4.2493 ± 0.0971 (17755) |
3.7260 ± 0.1201 (5321) *# |
4.1593 ± 0.1181 (14431) а |
4.2628 ± 0.1677 (18316) а |
The arithmetic mean ± the standard error for the log10 values of counts per minute (cpm) are shown; the geometric mean values of cpm calculated as the antilogarithms of the mean log10 cpm values are indicated in parentheses. * - p<0,05 to control group by Duncan's test; a - the same to the primary examination of patients; b - the same between groups during the re-examination of patients; # the same to cultures of the same group without agonist according to paired Student’s t test; n - number of observations.
In cultures of lymphocytes from apparently healthy control subjects, the addition of beta-adrenoreceptor agonist was found to suppress the proliferative response only at the optimal PHA concentration of 10 microgram/ml (Table 1). During the early traumatic period the sensitivity to the suppressive action of beta-adrenoreceptor stimulation was found to be considerably elevated. Hexoprenaline sulfate statistically significantly suppressed the lymphocyte proliferation within a whole range of mitogen concentrations. In the late traumatic period those alterations were retained against a background of conventional therapy despite the statistically significant elevation of the proliferation level as compared with the early traumatic period (primary examination of patients). On myelopidum inclusion in complex therapy the abolition of hexoprenaline suppressive effect was observed relatively to the preparation-free cultures, however, the proliferation level in cultures with a suboptimal mitogen concentration (2.5 microgram/ml) was statistically significantly reduced as compared with the control group.
The PHA-induced production of IFN-γ in cultures
The level of mitogen-induced production of IFN-γ in cultures with PHA was decreased in the early traumatic period (Table 2). Suppression of PHA-induced cytokine production was retained in the late traumatic period in patients of both groups. The level of IFN-γ production in cultures with PHA did not statistically differ from the mitogen-free cultures according to paired Student’s t test in patients with penetrating eye injury during the whole course of examination.
Table 2. Changes of IFN-g production in lymphocyte cultures with PHA in patients with penetrating eye injury (pg/ml).
Concent-ration of PHA,
micro-gram/ml |
Control group
(n=10) |
Primary examination of patients (n=20) |
Re-examination of patients |
myelopidum+
standard therapy (n=10) |
standard therapy (n=10) |
0 |
26.10 ± 5.22 |
38.60 ± 18.29 |
9.50 ± 2.14 |
5.50 ± 2.36 |
20 |
1699.40 ± 243.01# |
190.20 ± 124.40* |
211.30 ± 163.26* |
106.10 ± 94.72* |
* - p<0,05 to control group by Duncan's test; # - p<0,05 to cultures without mitogen by pair Student’s t-test; n - number of patients.
Changes of lymphocyte proliferative response in cultures with PWM
The level of PWM-induced lymphocyte proliferative response was decreased in the early traumatic period in 72-h cultures at the mitogen concentration of 5 microgram/ml, as compared with the control group (Table 3). Late traumatic period in patients receiving conventional therapy was marked by the elevation of proliferation level in cultures with 0.625 and 5.0 microgram/ml of mitogen, as compared with the primary examination, and in cultures with 0.625 microgram/ml, – relatively to the control group. On myelopidum inclusion the proliferative response was found to be increased versus the primary examination in all cultures with PWM. In 96-h cultures the elevation of lymphocyte proliferative response relatively to the parameters of nearly healthy volunteers was registered during the course of investigation when mitogen concentration was 10.0 microgram/ml, and with myelopidum inclusion it was also detected in cultures with 0.625 microgram/ml of PWM.
Table 3. Changes of lymphocyte proliferative response in cultures with PWM under penetrating eye injury.
Concentra-tion of PWM,
microgram/ml |
Control group
(n=10) |
Primary examination of patients (n=20) |
Re-examination of patients |
myelopidum+
standard therapy (n=10) |
standard therapy (n=10) |
72-h cultures |
0 |
3.5508 ± 0.0734 (3554) |
3.3148 ± 0.0707 (2064) |
3.2958 ± 0.0917 (1976)*b |
3.5649 ± 0.0787 (3672) |
0.625 |
4.0481 ± 0.1498 (11171) |
4.0831 ± 0.1114 (12108) |
4.4379 ± 0.0700 (27408)*а |
4.4484 ± 0.0687 (28077)*а |
1.25 |
4.3230 ± 0.0903 (21039) |
4.2644 ± 0.0839 (18381) |
4.5447 ± 0.0408 (35050)а |
4.4151 ± 0.0911 (26011) |
2.5 |
4.4418 ± 0.0852 (27658) |
4,3147 ± 0,0663 (20638) |
4,5531 ± 0,0359 (35739)а |
4,4781 ± 0,0853 (30068) |
5.0 |
4.5732 ± 0.0585 (37429) |
4.2900 ± 0.0706 (19497)* |
4.5914 ± 0.0486 (39031)а |
4.5035 ± 0.0583 (31880) а |
10.0 |
4.2395 ± 0.1260 (17360) |
4.1229 ± 0.1155 (13272) |
4.5294 ± 0.0474 (33836)а |
4.3424 ± 0.0654 (21999) |
96-h cultures |
0 |
3.4424 ± 0.1030 (2769) |
3.2812 ± 0.0627 (1911) |
3.2918 ± 0.0632 (1958) |
3.4867 ± 0.0636 (3067) |
0.625 |
4.0585 ± 0.1636 (11443) |
4.2646 ± 0.1111 (18392) |
4.6002 ± 0.0267 (39828)* |
4.2463 ± 0.0872 (17631) |
1.25 |
4.3147 ± 0.1341 (20640) |
4.3769 ± 0.0808 (23817) |
4.4890 ± 0.1357 (30831) |
4.4943 ± 0.1137 (31211) |
2.5 |
4.5640 ± 0.0901 (36645) |
4.4239 ± 0.0839 (26542) |
4.6675 ± 0.0385 (46508) |
4.5512 ± 0.0931 (35580) |
5.0 |
4.5671 ± 0.1464 (36908) |
4.4441 ± 0.0708 (27805) |
4.6670 ± 0.0965 (46448) |
4.5704 ± 0.0773 (37189) |
10.0 |
3.8863 ± 0.1672 (7696) |
4.2162 ± 0.0863 (16451)* |
4.6421 ± 0.0553 (43866)*а |
4.5136 ± 0.0640 (32631)* |
The arithmetic mean ± the standard error for the log10 values of counts per minute (cpm) are shown; the geometric mean values of cpm calculated as the antilogarithms of the mean log10 cpm values are indicated in parentheses. * - p<0,05 to control group by Duncan's test; a - the same to the primary examination of patients; b - the same between groups during the re-examination of patients; n - number of observations.
The detection frequency of antibodies to eye tissue antigens in blood plasma under penetrating eye injury
Due to the activation of thymus-dependent lymphocyte proliferative response in cultures with PWM in the late traumatic period it was essential to explore the possibility of the elevation of the antibody level to eye tissue antigens in suffered subjects. The increase in the detection frequency of antibodies to eye tissue antigens was not revealed (Table 4).
Table 4. Influence of myelopidum and conventional therapy on the detection frequency of antibodies to eye tissue antigens in blood plasma under penetrating eye injury.
Antigen |
Control group
(n=20) |
Re-examination of patients |
myelopidum+
standard therapy (n=27) |
standard therapy (n=23) |
BCP 54 |
0 (0) |
1 (3.7) |
2 (8.7) |
alfa-crystallin |
0 (0) |
1 (3.7) |
2 (8.7) |
S-antigen |
0 (0) |
1 (3.7) |
2 (8.7) |
with «+» reactions
to all antigens |
0 (0) |
1 (3.7) |
1 (4.3) |
with «+» reactions
to one antigen |
0 (0) |
1 (3.7) |
3 (13) |
n - number of patients; outside the parentheses indicate the number of positive results, in parentheses - the detection frequency as a percentage.
Dependence of the PHA-induced lymphocyte proliferative response on the endogenous cortisol level and sensitivity to beta-adrenergic regulation in vitro in the early period of penetrating eye injury
In the course of the analysis of individual parameters of the endogenous cortisol level in peripheral blood serum of sufferers (Figure 1) their considerable heterogeneity was revealed as compared with the control group according to F-test in the early traumatic period (F=8.43; p=0.003), as well as in the late traumatic period against a background of conventional therapy (F=4.79; p=0.026), while patients receiving myelopidum in therapy did not show differences in parameter variability relatively to control (F=0.86; p=0.846).
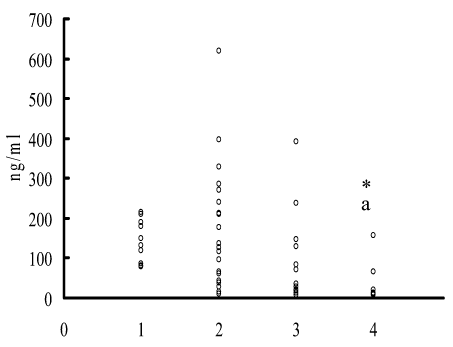
Figure 1. Change in the cortisol level in peripheral blood serum depending on a period of examination and type of therapy. On horizontal axis: 1 – control group; 2 – primary examination; 3 – conventional therapy; 4 – conventional therapy + myelopidum; on the vertical axis: cortisol concentration. * - p<0.05 to control; a – p<0.05 to primary examination.
Cortisol level in the late traumatic period in sufferers receiving additionally myelopidum comprised 34.94 ± 16.36 ng/ml (143.90 ± 16.70 in control; p<0.001), and for those receiving conventional therapy the value was 98.64 ± 33.38 (p>0.05 to control).
It should be indicated that primary examination of patients was carried out prior to conservative therapy, therefore marked heterogeneity in cortisol level as compared with the control group according to F-test in the early traumatic period points to individual peculiarities in response to trauma. In order to clarify the involvement of neuroendocrine stressor reactions in the suppression of lymphocyte proliferative response to PHA there was analyzed a dependence of its alteration in the early traumatic period on the endogenous cortisol level. All of patients were divided into subgroups depending on the level of this hormone following the values of clinical standard: 1 subgroup – high (over 216 ng/ml); 2 subgroup – normal (50-216 ng/ml), and 3 subgroup – low (under 50 ng/ml) level of endogenous cortisol.
The degree of suppression of the PHA-induced lymphocyte proliferative response in vitro without beta-adrenoreceptor agonist as compared to the normal value was found to depend on the concentration of endogenous cortisol (Table 5). The strongest suppression of proliferation was observed in patients with an increased or normal cortisol level. In patients with a decreased blood cortisol concentration, the lymphocyte proliferative response was not suppressed. When estimating how the sensitivity of the in vitro lymphocyte proliferative response to the immunosuppressive effect of the beta-adrenoreceptor agonist depended on the endogenous cortisol concentration, we found that the dependence was stronger in subjects with increased and normal cortisol levels than in those with a decreased level of the hormone.
Table 5. Dependence of PHA-induced lymphocyte proliferative response on the endogenous cortisol level and the effect of beta-adrenergic agonist hexoprenaline sulfate.
Con-centra-tion of PHA,
micro-gram/ml |
Cul-ture |
Control group
(n=10)
|
Groups of PEI patients |
with a high cortisol
level
(n = 6) |
with a normal cortisol
level
(n = 9) |
with a low cortisol
level
(n = 5) |
0 |
Without agonist |
3.5508 ± 0.0734
(3554) |
3.2854 ± 0.1017
(1929) |
3.3827 ± 0.0919
(2414) |
3.2278 ± 0.2115
(1690) |
|
With agonist |
3.6470 ± 0.0668
(4436) |
3.1508 ± 0.1408
(1415)* |
3.2959 ± 0.0592
(1977)* |
3.2679 ± 0.2000
(1853)* |
2.5 |
Without agonist |
3.8902 ± 0.1152
(7766) |
3.4015 ± 0.1743
(2521)*а |
3.4438 ± 0.0480
(2778)*а |
3.8676 ± 0.1831
(7372) |
|
With agonist |
3.8611 ± 0.0796
(7263) |
3.3980 ± 0.1602
(2500)*а |
3.1913 ± 0.0686
(1553) #*а |
3.7601 ± 0.1125
(5755) |
5.0 |
Without agonist |
4.0792 ± 0.1228
(12002) |
3.5435 ± 0.2168
(3496) *а |
3.4939 ± 0.0679
(3118) *а |
4.0860 ± 0.2187
(12191) |
|
With agonist |
4.0691 ± 0.1162
(11723) |
3.4578 ± 0.2404
(2870) *а |
3.2991 ± 0.0947
(1991) *а |
3.9667 ± 0.2344
(9262) |
10.0 |
Without agonist |
4.3980 ± 0.0695
(25003) |
3.6798 ± 0.1887
(4785) *а |
3.6719 ± 0.0897
(4698) *а |
4.1082 ± 0.2031
(12828) |
|
With agonist |
4.2272 ± 0.0915
(16873)# |
3.6476 ± 0.1763
(4442)* |
3.4403 ± 0.1165
(2756) #*а |
4.0060 ± 0.2302
(10140) |
20.0 |
Without agonist |
4.2991 ± 0.1000
(19919) |
4.0495 ± 0.1488
(11208) |
3.9635 ± 0.1375
(9195) |
4.1747 ± 0.1900
(14952) |
|
With agonist |
4.2493 ± 0.0971
(17755) |
3.7301 ± 0.2190
(5371) # |
3.5307 ± 0.1739
(3394) #*а |
4.0727 ± 0.2149
(11822) |
The arithmetic mean ± the standard error for the log10 values of counts per minute (cpm) are shown; the geometric mean values of cpm calculated as the antilogarithms of the mean log10 cpm values are indicated in parentheses. * - p<0.05 to control group by Duncan's test; a - the same to the group with a low cortisol level; # - the same to cultures of the same group without agonist according to paired Student’s t test; n - number of observations.
The effect of myelopidum inclusion in complex therapy of penetrating eye injuries on clinical manifestations of inflammation
It was established that the treatment of patients with myelopidum has led to faster decrease in clinical signs of inflammation and improvement of trauma process outcome (Table 6).
Table 6. Clinical efficacy of myelopidum inclusion in complex therapy of penetrating eye injuries.
The sign of clinical effect |
Myelopidum+
standard therapy |
Standard therapy |
14 day* |
28 day |
14 day |
28 day |
3 |
2 (7%) |
- |
9 (39%) |
3 (13%) |
4 |
18 (67%) |
- |
12 (52%) |
3 (13%) |
5 |
7 (26%) |
27 (100%) |
2 (9%) |
17 (74%) |
The number of patients is indicated, in parenthesis - % of the total amount of sufferers.
* - p=0.04 for the U-Mann-Whitney test to the 14-day study of group comparisons.
The data presented in this paper indicate that despite the local type of damage, the penetrating eye injury is characterized by the development of systemic changes in the immune system that depend on the period of traumatic process and on myelopidum inclusion in conventional therapy.
Early traumatic period prior to the anti-inflammatory therapy was marked by the decrease in lymphocyte proliferative response in cultures with PHA, and in PHA-induced production of IFN-γ. In the late traumatic period patients receiving conventional therapy demonstrated the elevation in lymphocyte blast transformation. The inclusion of myelopidum in therapy was found to extend the suppression of PHA-induced proliferative response of lymphocytes, did not abolish the IFN-γ production in cell cultures, and improved the anti-inflammatory effect of conventional therapy under the assessment of clinical effect of applied therapy. In earlier experiments [10,14] the appropriate phase changes in the level of the immune response under the penetrating eye injury were revealed that directly depended on the term of immunization. The suppression of the DTH reaction was observed in the early traumatic period while in the late traumatic period the DTH response was found to be activated. Similar dependence was also detected with other types of injury [15] and is apparently the manifestation of neuroendocrine stressor modulation of the immune response. Early period when the immunosuppression was registered, was simultaneous with the alarm reaction stage of the general adaptation syndrome that according to H. Selye [16] data was observed within 24-48 h from the moment of stressor action. Late period corresponded in time to a stage of resistance [16].
It was found that key role in eye immunopathology predominantly belonged to Th1-type reactions that in case of sympathetic ophthalmia resulted in the development of granulomatous inflammation in unaffected eye tissues [17]. In present work it was noticed that patients receiving myelopidum in complex therapy demonstrated the retaining of the suppression of PHA-induced proliferative response of lymphocytes and IFN-γ production, and according to experimental investigations [10] this was true to DTH suppression. In vitro the lymphocyte proliferative response in cultures with PHA was reduced under the effect of myelopeptides MP-1, MP-3, and MP-6 [18]. Introduction of MP-1, MP-3, MP-5, and MP-6 into peripheral blood mononuclear cell cultures resulted in a decrease in the level of proinflammatory cytokine IL-1b, and under the action of MP-5, it was detected both in IL-1b and TNF-a [19].
2021 Copyright OAT. All rights reserv
Earlier the decrease in the levels of lactoferrin, C-reactive protein, and IL-1b with the inclusion of myelopidum in complex therapy of PEI patients was revealed [20]. In case of experimental penetrating eye injury the inclusion of myelopidum in complex therapy resulted in a lowering of cell inflammatory infiltration in a scar and perifocal tissues, accelerated the formation of mature filamentous structures within the scar, and favored the epithelization of corneal damaged area in the early traumatic period [10]. Statistically significant decrease in inflammatory clinical manifestations following the myelopidum inclusion in complex therapy of PEI patients was demonstrated in present work.
As is demonstrated in the present work the lymphocyte proliferative response with thymus-dependent polyclonal activator of B-lymphocytes PWM was reduced in 72-h cultures during the early traumatic period. The level of lymphocyte proliferation in 96-h cultures with PWM, that occurs in the period being coincident with early PWM-induced IgM production [21] was found to be elevated. In the late traumatic period the increase in PWM-induced proliferative response of lymphocytes was observed both in 72-h and 96-h cultures. Those changes were mostly marked in patients receiving myelopidum in complex treatment. The ability of myelopeptides composing the myelopidum to promote the antibody formation is already well-known [8]. Following the experimental penetrating eye injury the myelopidum abolished the decrease in humoral response to sheep erythrocytes in rats in the early traumatic period, and restored it up to the level in unaffected animals, and did not influence the intensity of DTH suppression [10]. Due to that in our work it was found that the same patients retained the suppression of the proliferative response to T-cell mitogen (PHA) and IFN-γ production it was suggested a polarization of the type of the immune response from cell-mediated to humoral. Considering the key role of Th1-type reactions in the development of eye injury-induced complications [17] such polarization seems to be predicatively more favorable. As was determined in present work, the elevation of the detection frequency of antibodies to antigens BCP 54, a-crystalline, and S-antigen was lacking both under conventional therapy and under the inclusion of myelopidum in complex therapy.
Taking into account the involvement of neuroendocrine stressor reactions being developed in response to the threat of lacking in informatively important organ [3] in modulation of the immune system functions under penetrating eye injury, current work examined the change in sensitivity to beta-adrenergic agonist of in vitro PHA-induced proliferative response of lymphocytes and their interplay with the level of endogenous cortisol.
It was found that the sensitivity of the proliferative response to the suppressive effect of beta-adrenoreceptor stimulation was significantly increased in the early traumatic period. Hexoprenaline sulfate depressed the lymphocyte proliferation in a whole range of mitogen concentrations. Those alterations were retained in the late traumatic period against a background of conventional therapy. On myelopidum inclusion in complex therapy the reduction in the suppressive effect of hexoprenaline was observed relatively to agonist-free cultures.
Cortisol concentration in the late traumatic period in patients receiving the myelopidum in complex therapy was reduced that was related on the one hand with the use of dexamethasone in conventional therapy that caused the lowering in endogenous glucocorticoids on the principle of negative feedback, and on the other hand – with enhancement of this effect under the myelopidum action. It was demonstrated that individual peptides composing the myelopidum possessed marked opiate activity [22], and opiates were found to reduce the glucocorticoid synthesis and secretion [23].
It was shown in our work that early traumatic period prior to conservative therapy was characterized by significant heterogeneity in individual value of endogenous cortisol levels in peripheral blood sera of sufferers as compared with the control group. In 45% of sufferers the endogenous hormone concentration was within the normal values, in 30% of patients this parameter significantly exceeded those and corresponded to the values typical of strong stress, and in 25% of cases the concentration was essentially reduced.
Adequately to the direction of the cortisol concentration alteration it was revealed that the intensity of lymphocyte proliferative response was changed in the peripheral blood. Under the elevation or the lack in alteration of the endogenous hormone level marked suppression of lymphocyte proliferation in cultures with PHA was determined, and upon its decrease the proliferation intensity did not differ from the control value.
When estimating how the sensitivity of the in vitro proliferative response of the patients' lymphocytes to the immunosuppressive effect of the beta-adrenoreceptor agonist depended on the endogenous cortisol concentration, we found that the dependence was stronger in subjects with increased and normal cortisol levels than in those with a decreased level of the hormone. These differences were not unexpected, because there is a published evidence that glucocorticoids increase the sensitivity of target cells to beta-adrenergic agonists by affecting the density and affinity of beta-adrenoreceptors on the cell membrane and their coupling with G-proteins, increasing the receptor synthesis rate at the transcription level, and enhancing the transduction of a regulatory signal from them at the intracellular level [24]. Therefore, the combination of glucocorticoids with beta-adrenoreceptor agonists is widely used to potentiate their pharmacological effects in the treatment of a number of immunopathological states.
On the other hand, the immunosuppressive effect of glucocorticoids under the acute stress is substantially decreased by the pharmacological blockade of beta-adrenoreceptors, which indicates that their effect is mediated by endogenous catecholamines under the conditions of an increased functional expression of beta-adrenoreceptors [25]. In terms of the concept on two adaptation strategies [26], we may suggest the patients with increased and normal levels of endogenous cortisol to respond according to the resistance strategy (whose classical expressions are sympathoadrenal stress and general-adaptation syndrome); and the patients with a decreased level of the hormone, according to the tolerance strategy (a “concession” to environment, minimization of functions, and saving of reserves in difficult and dangerous situations). Regulatory signals mediated by alfa2-adrenoreceptors play an important role in the latter adaptation strategy; whereas beta-adrenoreceptors mediate metabolic processes synergistic to the glucocorticoid action, i.e., those characteristic of the resistance strategy [26]. Together with the published data on the role of ACAID [4-6], the results of our study indicate that glucocorticoids and the change in the sensitivity of the lymphocyte proliferative response to beta-adrenergic regulation being induced by them are involved in the development of immunosuppression during the early traumatic period of penetrating eye injury.
Thus, strong suppression of lymphocyte proliferation and high sensitivity to the immunosuppressive effect of a beta-adrenergic agonist in vitro are characteristic of the early post-injury period in PEI patients with an increased or normal endogenous cortisol level; in patients with a decreased level of this hormone, the lymphocyte proliferation is not suppressed, and the immunosuppressive effect of the agonist is decreased.
In conclusion, our results demonstrated an essential role of the elevation of the endogenous cortisol level and increase in cell sensitivity to beta-adrenergic regulation in the suppression of lymphocyte proliferative response in the early traumatic period prior to conservative therapy. The inclusion of myelopidum in complex therapy of sufferers favors the retaining of the suppression of PHA-induced proliferative response of lymphocytes, IFN-γ production in cultures, elevation of lymphocyte proliferation in cultures with thymus-dependent B-cell mitogen in the lack of the increase in the detection frequency of antibodies to eye tissue antigens, and faster reduction in the expressiveness of inflammatory clinical manifestations.
We thank Dr. Elena G. Mulmenko for critical reading of the manuscript and very helpful discussions, professor Sergey V. Sybiryak (deceased) for kind presentation of test-systems for immune-enzyme detection of antibodies to eye tissue antigens.
This study was supported by the Russian Foundation for Basic Research (project no. 10-04-96092r_ural_a and project no. 17-44-590995r_perm_a) and the programs of the Presidium of the Russian Academy of Sciences “Molecular and Cell Biology” and “Fundamental Science for Medicine”.
- Chereshneva MV, Shilov JuI, Badanina ON, Chereshnev VA, Kevorkov NN, Ponomareva TB, Shilov SJu (2001) Immunocorrection in Eye Injury. Ekaterinburg: Ural Branch of the Russian Academy of Sciences Publisher: 172 pp. (Monograph in Russian; ISBN 5-7631-1101-1).
- Lei F, Zhang J, Zhang J, He H, Du Y, Yang P (2008) A penetrating eye injury can affect the induction of anterior chamber-associated immune deviation. Mol Vis 14: 327-333. [Crossref]
- Gavrilova TV, Shilov JuI, Chuprina VV, Lobanova NL, Chereshneva MV, et al. (2009) Mechanisms of immune alterations and immunocorrection by myelopeptides under penetrating eye injury. Refract Surg Ophthalmol (Moscow) 4: 29-35 (in Russian).
- Treacy O, Fahy G, Ritter T, O'Flynn L (2016) Corneal immunosuppressive mechanisms, Anterior chamber-associated immune deviation (ACAID) and their role in allograft rejection. Methods Mol Biol 1371: 205-214. [Crossref]
- Taylor AW (2016) Ocular immune privilege and transplantation. Front Immunol 7: 37. [Crossref]
- Stein-Streilein J (2013) Mechanisms of immune privilege in the posterior eye. Int Rev Immunol 32: 42-56. [Crossref]
- Yanoff M, Duker JS, Augsburger JJ (2003) Editors, Ophthalmology, 2nd ed. Mosby.
- Mikhailova A, Fonina L, Kirilina E, Gur'yanov S, Efremov M, et al. (2003) Peculiarities of immunocorrective effects of the bone marrow regulatory peptides (myelopeptides). Regul Pept 114:183-187. [Crossref]
- Mikhailova AA, Belevskaya RG, Kalyuzhnaya M, Fonina LA, Liashenko VA, et al. (2006) Myelopeptide-2 recovers interleukin-2 synthesis and interleukin-2 receptor expression in human T lymphocytes depressed by tumor products or measles virus. J Immunother 29: 306-312. [Crossref]
- Berkasova NL, Gavrilova TV, Shilov JuI, Chereshneva MV, Medvedeva SJ, et al. (2008) Effects of myelopeptides on immune response and morphometrical manifestations of inflammation in experimental penetrating wound of the eye. Bull Exp Biol Med 145:341-343.
- Yakovleva VG, Sibiryak SV, Golovin VP, Kireev VL (1998) Isolation and purification rodopsinkinazy (retinal S-antigen). In: Proceedings of Conference of Biochemists of the Urals and Western Siberia, Ufa: 273-276 (in Russian).
- Yakovleva VG, Sibiryak SV, Golovin VP (2000) Enzyme-linked immunosorbent test system for detection of antibodies to arrestin and its use in ophthalmo-specific immunological monitoring. Med Immunol (St. Petersburg) 2: 206-207 (in Russian).
- Aznabaev MT, Speranskiĭ VV, Aznabaev RA, Golovin VP, Yakovleva VG, et al. (2000) Immunological status and its changes in children during surgery on anterior eye segment. Vestn Oftalmol 116: 21-25 (in Russian).
- Chereshnev VA, Faizrakhmanov RR, Gavrilova TV, Chereshneva MV, Shilov JuI, et al. (2007) The use of Profetal for correction of the stress- and trauma-induced changes in the immune response against a xenogeneic antigen in rats with a penetrating wound of the eye. Dokl Biol Sci 417: 420-422. [Crossref]
- Chereshnev VA, Kevorkov NN, Shmagel KV, Yarilin AA (1997) Immunology of combined radiation injury. Ekaterinburg: Ural Branch of the Russian Academy of Sciences: 164 pp. (Monograph in Russian; ISBN 5-7691-0711-1)
- Selye H (1936) A syndrome produced by diverse nocuous agents. Nature 138: 32-32. [Crossref]
- Furusato E, Shen D, Cao X, Furusato B, Nussenblatt RB, et al. (2011) Inflammatory cytokine and chemokine expression in sympathetic ophthalmia: a pilot study. Histol Histopathol 26:1145-1151. [Crossref]
- Gavrilova TV, Gein SV, Pogudina TA, Chereshnev VA (2005) Mechanism underlying the effect of myelopeptides on lymphocyte proliferation in vitro. Bull Exp Biol Med 140: 74-76.
- Chereshnev VA, Gein SV, Mazunina LS, Gavrilova TV, Chereshneva MV (2011) Myelopeptides regulate the microbicidic and secretory activities of innate immunity effectors. Dokl Biochem Biophys 436: 53-55. [Crossref]
- Gavrilova TV, Chuprina VV, Davydova EV, Shilov JuI, Chereshneva MV, et al. (2008) Immunomodulatory action of myelopidum under its inclusion in complex therapy of patients with penetrating ocular injuries. Med Immunol (St. Petersburg) 10: 239-244 (In Russian).
- Limburg PC, Hummel-Tappel E, Oosterhuis HJ, The TH (1985) In vitro T-cell dependent B-cell activity in myasthenia gravis. Clin Exp Immunol 61: 31-38. [Crossref]
- Petrov RV, Mikhailova AA, Fonina LA, Stepanenko RN (1999) Myelopeptides. Singapore: World Scientific: 181 pp.
- Chereshnev VA, Geĭn SV (2009) Beta-endorphin as the endogenous regulator of immune processes. Ross Fiziol Zh Im I M Sechenova 95: 1279-1290. (In Russian)
- Taylor DR, Hancox RJ (2000) Interactions between corticosteroids and beta agonists. Thorax 55: 595-602.
- Shilov SJu, Shilov JuI, Chereshnev VA (2004) Modification of the immunomodulating effect of hydrocortisone under the conditions of beta-adrenoreceptor blockage. Dokl Biol Sci 396: 200-202.
- Kulinskii VI, Ol’khovskii IA (1992) Two adaptive strategies in adverse conditions - resistant and tolerant. The role of hormones and receptors. Usp Sovrem Biol 112: 697-714 (in Russian).

