Abstract
Sarcopenia has been identified as a serious risk factor for morbidity and mortality in late aging and early aging related to neurological disorders, in particular SCI. In the presented paper, the quantitative potential of computed tomography (CT) image analysis is used to describe skeletal muscle quality changes of anatomically defined human skeletal muscles. Current literature reports average Hounsfield unit (HU) values and/or segmented soft tissue cross-sectional areas to investigate muscle quality and quantity. Standardized methods for CT analyses and their utility as comorbidity indexes remain undefined, and no existing studies compare these methods to the assessment of entire radio densitometric distributions. The primary aim of this study is to present a comparison of material content of entire radio densitometric muscle distributions. The results highlight the specificities of each muscle quality metric between an able-bodied subject, an elderly subject and and SCI subject, and particularly highlight the value of the connective tissue regime in this regard.
Introduction
The progressive decay of aging muscle, known as Sarcopenia, has been consistently identified as a risk factor for morbidity and mortality [1–7]. Its prevalence in older persons and neurological patients, in particular Spinal Cord Injury (SCI) is characterized by serious decreases in both physical activity and muscle masses, but a quantitative definition for its diagnosis remains debated [8–11]. Clinical literature correlates decay of physiological function (loss of muscle strength) to sarcopenia [12–14]. However, the degree to which this loss of muscle strength may be attributed to the loss of muscle mass remains uncertain, if quality of skeletal muscle is not clearly defined and quantitate [15–18]. Nonetheless, methodological comparisons for the precise, non-invasive quantification of the progressive reduction of muscle quality remain disparately described in literature. Standardizing a quantitative methodology for muscle assessment in this regard would allow sarcopenia concept research to become a useful indicator, in particular of compensatory targets for clinical intervention. Aging skeletal muscle has a significantly reduced proportion of glycolytic type II muscle fibers compared to young muscle, that may explain at least in part its decreased speed, force and, thus, power [19-21]. Additionally, aged tissues significantly lack the ability to process triglycerides, resulting in increased lipid droplet storage in and along muscle fibers [22]. This increased adiposity and decreased contractility has been linked to mitochondrial dysfunction and impaired oxidative metabolism, which has been shown to relate to metabolic insulin resistance and Type 2 diabetes mellitus in patients [23, 24]. In general, increased percentage of non-contractile tissues (adipocytes and fibrous connective tissues), aggravates the size loss (and eventually, number) of muscle fibers, conferring an increased risk for reduced mobility, frailty, disability, and eventual hospitalization [25 - 27]. Studying how these changes affect mobility is the prime motive for lower extremity function (LEF) research, which cites LEF as the main indicator for mobility as a clinical screening tool [28]. LEF is generally assessed by measuring walking capacity (gait speed) and leg strength [29]. Altogether, the association of sarcopenic muscle degeneration with decreasing LEF illustrates how aging induces mobility impairment, incident disability, and eventual mortality [30–34].
Muscle biopsy is the standard clinical procedure used for the assessment of muscle, but the procedure is invasive and occasionally limited in relevance by the small size of excised tissue. However, recent investigations have realized the potential of X-ray computed Tomography (CT) and Magnetic Resonance Imaging (MRI) to describe muscle quality and composition. This is often performed either quasai-quantitatively, via the visual grading of muscle structure morphologies [35–38], or quantitatively via the computation of muscle cross-sectional areas and radiodensitometric absorption values in CT, measured in Hounsfield units (HU) [39–44]. Despite the superior soft tissue contrast in MRI and non-dependence on the use of ionizing radiation, CT has higher spatial resolution and is comparatively less obfuscated by technical variations in machine preparation and acquisition protocols [45]. These notions are critical when attempting to discern diagnostically-relevant information from cross-sectional images of soft tissue.
Recent studies have demonstrated the utilization of CT image analysis to quantify muscle composition and quality [46]. Analyzing muscle degeneration in particular has been of great focus in research and many studies have been focused how changes in skeletal muscle density correlate with changes in muscular volume and function in patients suffering from a variety of conditions or diseases. [47]. Further research is therefore essential to associate how muscle composition can give indications about the overall physical condition of the patient.
Methods
Patients were CT scanned using a phantom that calibrated against the density changes within each tissue. The muscle that was identified was the Tibialis Anterior. The scans ranged from the proximal tibia to the lateral malleolus. Three subjects were recruited, a young able-bodied control subject, an elderly subject and a spinal cord injury patient. The scans were segmented using Mimics (Materialize) using masking technique to identify the Tibialis Anterior, the tibia and the fibula in each slice. Three dimensional models were created from the masks. Depending on the Hounsfield values for the muscle, the pixel was determined to belong to fat (HU<-10), connective tissue (-10<HU<41) and muscle tissue (HU>41). By examining the density distributions in the three-dimensional model, the material composition of the muscle was quantified.
Results
The linear relationship between the Hounsfield units and the material density was used to map all voxels belonging to the Tibialis Anterior muscle. A three-dimensional image of the muscle and the density values was created for all the subjects and can be seen in Figure 1. The figure shows the three-dimensional model of the muscle along with the representation of the three different tissue types: fat, connective tissue and muscle fibres. The material distributions were quantified for both legs which gives information about the symmetrical aspects between the muscles. From the figure, it can be seen how the elderly subject and pathological subject, who suffered from asymmetrical lower body paralysis from a pelvic mass infiltration of the sciatic nerve, exhibited increasing amounts of fat and loose connective tissue, compared to the control subject. Likewise, the left leg of the pathological subject contains higher amount of fat than the right leg, thus quantifying the asymmetric nature of the subject’s condition and be able to quantify the severity of the muscle degeneration. Figure 2 shows the histogram analysis of the soft material content of the entire leg for the same individuals. The graphs show the volume as a function of Hounsfield units. The height of the peak and the location give indication about amount of each tissue present in the thigh. From the figure, it can be seen the two peaks accounting fat and muscle and how their distribution is dramatically different in three cases. The comparison between figure 1 and 2 shows the different composition of a single muscle (Fig. 1) in respect of the overall thigh (histograms in Fig. 2). Muscle and fat are inverted in terms of volumes for young healthy and aged individual while the distribution in the pathological subject has a completely different profile.
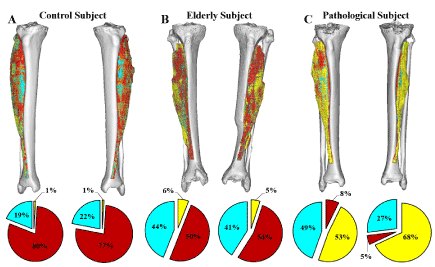
Figure 1. Shows the material composition within the Tibialis anterioris from each subject. The tissue types are: fat (yellow), connective tissue (cyan), and muscle (red).
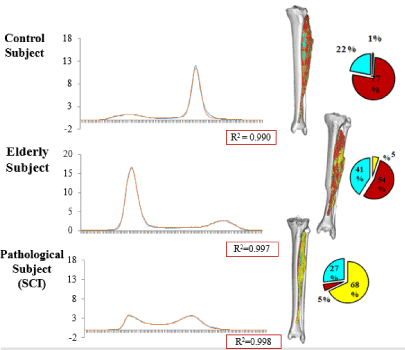
Figure 2. Material histogram distribution of the right leg between all the subjects
Discussion
This work shows the possibility to characterize graphically the muscle based on computer tomographic image and to create a subject specific profile that could be used to assess sarcopenia. The results show how the material changes in the muscle. Understanding of how the distribution between muscle tissue, fat and connective tissue can underline how sarcopenia occurs in patients. Connection with the biomechanical output of the muscle has been established [47], where a higher muscle tissue peak and a lower fat tissue peak were associated with increased kinetic performance of the muscle. This is in agreement with the findings presented in the study where a reduction of muscle tissue was found in the elderly subject and the SCI patient compared to the able-bodied subject [48-59]. Using the analysis presented in the paper to quantify the material composition of any anatomically defined skeletal muscle is the first step to standardize when discussing how to address skeletal muscle quality and rehabilitation by Assisted Exercise.
References
- Metter EJ, Talbot LA, Schrager M, Conwit R (2002) Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359-365. [Crossref]
- Rantanen T, Harris T, Leveille SG, Visser M, Foley D, et al. (2000) Muscle strength and body mass index as long-term predictors of mortality in initially healthy men. J Gerontol A Biol Sci Med Sci 55: M168-173. [Crossref]
- Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, et al. (2006) Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci 61: 72-77. [Crossref]
- Goodpaster BH, Carlson CL, Visser M, Kelley DE, Scherzinger A, et al. (2001) Attenuation of skeletal muscle and strength in the elderly: The Health ABC Study. J Appl Physiol (1985) 90: 2157-2165. [Crossref]
- Volpi E, Nazemi R, Fujita S (2004) Muscle tissue changes with aging. Curr Opin Clin Nutr Metab Care 7: 405-410. [Crossref]
- Lynch GS (2001) Therapies for improving muscle function in neuromuscular disorders. Exercise and Sport Sciences Reviews. 29: 141–148.
- Kalyani RR, Corriere M1, Ferrucci L2 (2014) Age-related and disease-related muscle loss: the effect of diabetes, obesity, and other diseases. Lancet Diabetes Endocrinol 2: 819-829. [Crossref]
- Baumgartner RN, Koehler KM, Gallagher D, Romero L, Heymsfield SB, et al. (1998) Epidemiology of sarcopenia among the elderly in New Mexico. Am J Epidemiol 147: 755-763. [Crossref]
- Janssen I, Heymsfield SB, Ross R (2002) Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 50: 889–896. [Crossref]
- Newman AB, Kupelian V, Visser M, Simonsick E, Goodpaster B, Nevitt M, et al. (2002) Sarcopenia: Alternative Definitions and Associations with Lower Extremity Function. J Am Geriatr Soc, 51: 1602–1609. [Crossref]
- Kern H, Hofer C, Loefler S, Zampieri S, Gargiulo P, et al. (2017) Atrophy, ultrastructural disorders, severe atrophy and degeneration of denervated human muscle in SCI and Aging. Implications for their recovery by Functional Electrical Stimulation, updated 2017. Neurol Res 39:660-666.
- Brooks SV, Faulkner JA (1994) Skeletal muscle weakness in old age: underlying mechanisms. Med Sci Sports Exerc. 26: 432. [Crossref]
- Maughan RJ, Watson JS, Weir J (1983) Strength and cross-sectional area of human skeletal muscle. J Physiol 338: 37-49. [Crossref]
- Reed RL, Pearlmutter L, Yochum K, Meredith KE, Mooradian AD (1991) The relationship between muscle mass and muscle strength in the elderly. J Am Geriatr Soc. 39: 555–561.
- Jubrias SA, Odderson IR, Esselman PC, Conley KE (1997) Decline in isokinetic force with age: muscle crosssectional area and specific force. Pflugers Arch. 434: 246–253. [Crossref]
- Kern H, Carraro U (2014) Home-Based Functional Electrical Stimulation for LongTerm Denervated Human Muscle: History, Basics, Results and Perspectives of the Vienna Rehabilitation Strategy. Eur J Transl Myol 24:3296.
- Overend TJ, Cunningham DA, Kramer JF, Lefcoe MS, Paterson DH (1992) Knee extensor and knee flexor strength: cross-sectional area ratios in young and elderly men. J Gerontol 47: M204-210. [Crossref]
- Young A, Stokes M, Crowe M (1984) Size and strength of the quadriceps muscles of old and young women. Eur J Clin Invest 14: 282-287. [Crossref]
- Larsson L, Sjödin B, Karlsson J (1978) Histochemical and biochemical changes in human skeletal muscle with age in sedentary males, age 22–65 years. Acta Physiol Scand. 103: 31–39. [Crossref]
- Larsson L, Li X, Frontera WR (1997) Effects of aging on shortening velocity and myosin isoform composition in single human skeletal muscle cells. Am J Physiol. 272(2 Pt 1): C638–C649. [Crossref]
- Carraro U, Kern H, Gava P, Hofer C, Loefler S, et al. (2015) Biology of Muscle Atrophy and of its Recovery by FES in Aging and Mobility Impairments: Roots and By-Products. Eur J Transl Myol. 25: 221-30. [Crossref]
- Lang T, Streeper T, Cawthon P, Baldwin K, Taaffe DR, Harris TB (2010) Sarcopenia: etiology, clinical consequences, intervention, and assessment, Osteoporos Int. 2010; 21: 543-559. [Crossref]
- Scott D, de Courten B, Ebeling PR (2016) Sarcopenia: a potential cause and consequence of type 2 diabetes in Australia's ageing population? Med J Aust 205: 329-333. [Crossref]
- Goodpaster BH, Thaete FL, Kelley DE (2000) Thigh adipose tissue distribution is associated with insulin resistance in obesity and in type 2 diabetes mellitus. Am J Clin Nutr. 71:885–892. [Crossref]
- Janssen I, Shepard DS, Katzmarzyk PT, Roubenoff R (2004) The healthcare costs of sarcopenia in the United States. J Am Geriatr Soc 52: 80-85. [Crossref]
- Kent-Braun JA, Ng AV (2000) Skeletal muscle oxidative capacity in young and older women and men. J Appl Physiol (1985) 89: 1072-1078. [Crossref]
- Buford TW, Lott DJ, Marzetti E, Wohlgemuth SE, Vandenborne K, Pahor M, et al. Age-related differences in lower extremity tissue compartments and associations with physical function in older adults. Exp Gerontol. 2012; 47(1):38–44. https://doi.org/10.1016/j.exger.2011.10.001 PMID: 22015325
- Rantanen T, Guralnik JM, Ferrucci L, Leveille S, Fried LP (1999) Coimpairments: strength and balance as predictors of severe walking disability. J Gerontol A Biol Sci Med Sci 54: M172-176. [Crossref]
- Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, et al. (2003) Physical performance measures in the clinical setting. J Am Geriatr Soc 51: 314-322. [Crossref]
- Chang M, Saczynski JS, Snaedal J, Bjornsson S, Einarsson B, et al. (2013) Mid-life physical activity preserves lower extremity function in older adults: Age Gene/Environment Susceptibility (AGES)–Reykjavik Study. J Am Geriatr Soc. 61: 237–242.
- Gava P1, Kern H, Carraro U (2015) Age-associated power decline from running, jumping, and throwing male masters world records. Exp Aging Res 41: 115-135. [Crossref]
- Guralnik JM1, Ferrucci L, Simonsick EM, Salive ME, Wallace RB (1995) Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med 332: 556-561. [Crossref]
- Guralnik JM, Simonsick EM, Ferrucci L, Glynn JR, Berkman LF, et al. (1994) A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol. A Biol Sci Med Sci. 49:M85–M94
- Cooper R, Kuh D, Hardy R (2010) Objectively measured physical capability levels and mortality: systematic review and meta-analysis. BMJ (Clinical Research Ed.). 341, c4467.
- Sayer AA, Robinson SM, Patel HP, Shavlakadze T, Cooper C, et al. (2013) New horizons in the pathogenesis, diagnosis and management of sarcopenia. Age Ageing 42: 145-150. [Crossref]
- Swash M, Brown MM, 2021 Copyright OAT. All rights reservd the clinical assessment of neuromuscular disease. Muscle Nerve 18: 708-714. [Crossref]
- Edmunds KJ, Gíslason MK, Arnadottir ID, Marcante A, Piccione F, et al. (2016) Quantitative Computed Tomography and Image Analysis for Advanced Muscle Assessment. Eur J. Transl. Myol. 26: 93–100. [Crossref]
- Fischer D, Kley R, Strach K, Meyer C, Sommer T, et al. (2008) Distinct muscle imaging patterns in myofibrillar myopathies. Neurology. 71:758–65. [Crossref]
- Mercuri E, Talim B, Moghadaszadeh B, Petit N, Brockington M, et al. (2002) Clinical and imaging findings in six cases of congenital muscular dystrophy with rigid spine syndrome linked to chromosome 1p (RSMD1). Neuromuscul Disord. 12:631–638. [Crossref]
- Carraro U, Boncompagni S, Gobbo V, Rossini K, Zampieri S, et al. (2015) Persistent Muscle Fiber Regeneration in Long Term Denervation. Past, Present, Future. Eur J Transl Myol 25: 4832. [Crossref]
- Gargiulo P, Kern H, Carraro U, Ingvarsson P, Knútsdóttir S, et al. (2010) Quantitative color three-dimensional computer tomography imaging of human long-term denervated muscle. Neurol Res. 32: 13.
- Helgason T, Gargiulo P, Jóhannesdóttir F, Ingvarsson P, Knútsdóttir S, et al. (2005) Monitoring muscle growth and tissue changes induced by electrical stimulation of denervated degenerated muscles with CT and stereolithographic 3D modeling. Artif Organs. 29: 440–443. [Crossref]
- Snijder M, Visser M, Dekker J, Goodpaster B, Harris T, et al. (2005) Low subcutaneous thigh fat is a risk factor for unfavourable glucose and lipid levels, independently of high abdominal fat. The Health ABC Study. Diabetologia. 48:301–308. [Crossref]
- Mah P, Reeves TE, McDavid WD (2010) Deriving Hounsfield units using grey levels in cone beam computed tomography. Dento Maxillo Facial Radiology. 39: 323–35. [Crossref]
- Carraro U, Edmunds KJ, Gargiulo P (2015) 3D False Color Computed Tomography for Diagnosis and Followup of Permanently Denervated Human Femoral Muscles Submitted to Functional Electrical Stimulation. Eur J Transl Myol. 25: 129–140.
- Edmunds KJ, Gíslason MK, Arnadottir ID, Marcante A, Piccione F, et al. (2016) Quantitative Computed Tomography and Image Analysis for Advanced Muscle Assessment. Eur J Transl Myol 26: 6015. [Crossref]
- Edmunds K, Gíslason M, Sigurðsson S, Guðnason V, et al. (2018) Advanced quantitative methods in correlating sarcopenic muscle degeneration with lower extremity function biometrics and comorbidities. PLoS One 13: e0193241. [Crossref]
- Kern H, Barberi L, Löfler S, Sbardella S, Burggraf S, et al. (2014) Electrical stimulation counteracts muscle decline in seniors. Front Aging Neurosci. 6:189. [Crossref]
- Zampieri S, Pietrangelo L, Loefler S, Fruhmann H, Vogelauer M, et al. (2015) Lifelong Physical Exercise Delays Age-Associated Skeletal Muscle Decline. J Gerontol A Biol Sci Med Sci. 70:163-73. [Crossref]
- Zampieri S, Mosole S, Löfler S, Fruhmann H, Burggraf S, et al. (2015) Physical Exercise in Aging: Nine Weeks of Leg Press or Electrical Stimulation Training in 70 Years Old Sedentary Elderly People. Eur J Transl Myol 25: 237-242. [Crossref]
- Zampieri S, Mammucari C, Romanello V, Barberi L, Pietrangelo L, et al. (2016) Physical exercise in aging human skeletal muscle increases mitochondrial calcium uniporter expression levels and affects mitochondria dynamics. Physiol Rep. 4(24). [Crossref]
- Carraro U, Gava K, Baba A, Piccione F, Marcante A (2016) Fighting muscle weakness in advanced aging by take-home strategies: Safe anti-aging full-body in-bed gym and functional electrical stimulation (FES) for mobility compromised elderly people. Biol Eng Me. 1: 1-4.
- Carraro U, Gava K, Musumeci A, Baba A, Piccione F, et al. (2018) Safe Antiaging Full-Body In-Bed Gym and FES for Lazy Persons: Home In-Bed Exercises for Fighting Muscle Weakness in Advanced Age. In Springer-Nature Book: Rehabilitation Medicine for Elderly Patients, Masiero S, Carraro U, Eds., 2018; pag. 43-52.
- Carraro U, Gava K, Baba A, Marcante A, Piccione F, et al. To contrast and reverse skeletal muscle atrophy by Full-Body In-Bed Gym, a mandatory life-style for older olds and borderline mobility impaired persons. Springer-Nature Book: Muscle Atrophy, Junjie Xiao, Ed., in press.
- Barber L1, Scicchitano BM2, Musaro A3 (2015) Molecular and Cellular Mechanisms of Muscle Aging and Sarcopenia and Effects of Electrical Stimulation in Seniors. Eur J Transl Myol 25: 231-236. [Crossref]
- Sajer S1 (2017) Mobility disorders and pain, interrelations that need new research concepts and advanced clinical commitments. Eur J Transl Myol 27: 7179. [Crossref]
- Paillard T1 (2018) Muscle plasticity of aged subjects in response to electrical stimulation training and inversion and/or limitation of the sarcopenic process. Ageing Res Rev 46: 1-13. [Crossref]
- Albertin G, Kern H, Hofer C, Guidolin D, Porzionato A, et al. (2018) Two years of Functional Electrical Stimulation by large surface electrodes for denervated muscles improve skin epidermis in SCI. Eur J Transl Myol. 28: 7373.
- Albertin G, Hofer C, Zampieri S, Vogelauer M, Löfler S, et al. (2018) In complete SCI patients, long-term functional electrical stimulation of permanent denervated muscles increases epidermis thickness. Neurol Res. 40: 277-282. [Crossref]


