Abstract
Background: Growth factor cocktail (GFC) treatment in combination with microneedling in patients with androgenetic alopecia (AGA) is effective and safe. However, there is a lack of studies on therapeutic effects of growth factor cocktail including fibroblast growth factor 9 (FGF9) in patients with androgenetic alopecia.
Objective: The aim of this study is to evaluate the efficacy of GFC including FGF9 (Cellcurin) on AGA.
Methods: The study was performed on AGA patients who were treated with topical Cellcurin in combination with microneedling once in 2 weeks for 3 months. The scalp was divided into two sides as right and left and treated with Cellcurin (right side) and normal saline (left side), respectively; both the sides were treated with microneedle depth of 0.5 mm. A total of 18 subjects (nine males and nine females) were enrolled in the present study. Cellcurin was topically applied using a microneedle medical device. Treatment efficacy was evaluated by phototrichogram and digital photograph analysis after six repeated treatments for 3 months.
Results: Phototrichogram of Cellcurin and normal saline treated side on scalp showed 27.1/cm2 and 5.4/ cm2 increase in hair density and 2.7µm and -0.6µm change in hair diameter, respectively. These results were statistically significant (p<0.05) except for hair diameter in the normal saline side. With respect to density and diameter, Cellcurin was significantly more effective in imparting its effect than normal saline (p<0.05). Cellcurin treatment was compared with SGF57 which is a GFC without FGF9, and was found to be more effective in increasing hair density as compared to SGF57 (p<0.05).
Conclusion: Cellcurin treatment with microneedling was found to be effective and safe and seemed to be more effective than SGF57. Further research is necessary to confirm the results of the present study and to determine the most effective factor of GFC.
Key words
Androgenetic alopecia, Cellcurin, Fibroblast growth factor 9, Growth Factor Cocktail
Introduction
Androgenetic alopecia (AGA) is the most common type of hair loss, which is caused by a combination of male hormonal factors and genetic factors, and includes male pattern hair loss (MPHL) and female pattern hair loss (FPHL) [1]. The typical features are the changes in hair cycle accompanied with progressive decrease in hair follicles in anagen phase, and inversely increase in hair follicles in telogen phase, as well as miniaturization of hair follicles [1]. As a result, conversion of terminal hairs into vellus hairs and increase in hair loss takes place [2].
Treatment of AGA includes therapeutic treatment with drugs and hair transplantation, and the treatment option is determined depending on the patient’s age, progression of AGA, and prognosis of the treatment [2]. Oral administration of finasteride and topical application of minoxidil are still the most common approaches. Recently, due to the limited study of finasteride and minoxidil for the long-term efficacy and safety, new treatment approaches such as platelet-rich plasma, growth factors and cytokines are under active investigation [3]. Lin et al. reported that fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice [4]. There are many other reports stating that growth factors are involved in the hair cycle and stimulate regeneration of hairs; however, clinical reports are scarce. Lee et al. reported the efficacy of growth factor cocktail (GFC) treatment in combination with microneedle therapy, but the study was only conducted for 5 weeks period [5]. Recently, our group reported similar approach with treatment period of 3 months [6].
Importance of fibroblast growth factor 9 (FGF9) in hair follicle regeneration has previously been reported [8]. Nevertheless, there is a lack of studies on FGF9 as a GFC component in human clinical trial. Based on previous reports, we investigated the therapeutic effect of GFC with FGF9 (Cellcurin) in AGA patients.
Materials and methods
Patient groups
A total of 18 patients with mild to moderate AGA were recruited for the present study after obtaining informed consent. We excluded the patients treated for AGA within a year and with infectious disease, immunodeficiency, and keloid history. Nine male patients aged between 28 and 52 years with male pattern hair loss (MPHL) II, III, IV, V or VI according to Norwood-Hamilton grading scale and nine female patients aged between 27 and 54 years old with female pattern hair loss (FPHL) II according to Ludwig scale were enrolled in the study. This study was approved by the Institutional Review Board of Myongji Hospital, and performed according to the guidelines of the Declaration of Helsinki.
Materials and treatment regimens
A 3-month split study was conducted at the Department of Dermatology, Seonam University College of Medicine, Myongji Hospital, Goyang, Korea from May to October 2015. All subjects underwent GFC treatment including FGF9 with microneedling, right side was treated with GFC and left side was treated with normal saline. Depth of microneedling was 5 mm and no other treatments such as topical application of minoxidil solution or systemic administration of finasteride or dutasteride were conducted.
Growth Factor Cocktail
The GFC used in this study (Cellcurin; PnP Biopharm, Seoul, Korea) consisted of basic fibroblast growth factor (FGF, 2.5 μg/mL), vascular endothelial growth factor (VEFG, 2.5 μg/mL), keratinocyte growth factor-2 (KGF-2, 2.5 μg/mL), stem cell factor (SCF, 2.5 μg/mL), insulin-like growth factor-1 (IGF-1, 1.25 μg/mL), fibroblast growth factor 9 (FGF9, 2.5 μg/mL), superoxide dismutase-1 (SOD-1, 5 μg/mL), and noggin peptide (10 μg/mL).
Microneedle
The microneedle device (FN-1; Woorhimeca, Gyeonggi-do, Korea) consists of nine 32-gauge microneedles with automatic movements in the vertical direction and the needle depth is adjustable from 0.1 to 2.0 mm.
Treatment
Cellcurin with microneedling was applied on right side of the scalp and normal saline with microneedling was applied on left side of the scalp. Both the sides were treated with microneedle depth of 0.5 mm (Figure 1). Cellcurin, provided as a freeze-dried powder, was dissolved in 5 ml normal saline prior to use. Approximately 2.5 ml of Cellcurin solution was topically applied to the scalp with microneedling. Each patient received six treatments at intervals of 2 weeks for a period of 3 months.
Figure 1. Schematic diagram of scalp divided as right and left sides for split test.
Measurement of parameters
Before the treatment, individual patient’s scalp was divided into right and left sides and both the sides of the scalp were marked by a tattoo to ensure hair’s reproducibility. Phototrichogram (Folliscope 2.8, Lead M, Seoul, Korea) was taken from a fixed area marked with a tattoo on both the sides to measure hair density and diameter at the baseline, at the end of treatment and at 3 months later.
Statistical analysis
Wilcoxon matched-pairs signed-rank test (p-value<0.05) was performed using STATA/SE ver.12 to test the effectiveness of treatment, before and after the treatment on same patients. Student’s t-test (p-value<0.05) was conducted to analyze the difference between the effect of treatment on the right and left sides of the scalp.
Results
Characteristics of patients
A total of 18 patients with mean age of 41.1 ± 8.03 years completed the 3-month study. Of the 18 patients, nine were males with mean age of 44.8 ± 8.1 years and nine were females with mean age of 37.3 ± 8.2 years. The nine MPHL patients were classified as 1 type II, 3 type III, 1 type IV, 2 type V, and 2 type VI. The nine FPHL patients were classified as 9 type II (Table 1).
Table 1. Baseline characteristics of patients enrolled in the present study.
|
|
Total |
Male |
Female |
Patients |
|
18 |
9 |
9 |
Mean Age (years) |
|
41.1 ± 8.03 |
44.8 ± 8.1 |
37.3 ± 8.2 |
Pattern of Hair Loss |
|
|
|
|
|
MPHL II |
|
1 |
|
|
MPHL III |
|
3 |
|
|
MPHL IV |
|
1 |
|
|
MPHL V |
|
2 |
|
|
MPHL VI |
|
2 |
|
|
FPHL II |
|
|
9 |
Efficacy assessment
All patients were treated with Cellcurin with microneedling once in 2 weeks for 3 months. Phototrichogram and photography were taken from the right and left sides of the patient’s scalp at the baseline and at the end of treatment.
The efficacy of GFC treatment with microneedling
The density and diameter of hair were measured at baseline and after 3 months of treatment. Phototrichogram showed that the Cellcurin treatment with microneedling increased the density and diameter of hair on right (Cellcurin) and left (normal saline) side of the scalp (Figure 2). The density of hair on the right and left sides of the scalp was increased by 27.1/cm2 and 5.4/cm2, respectively and the diameter of hair on the right and left sides of the scalp was increased by 2.72 μm and -0.61 μm, respectively with statistical significance (Table 2). Photography also revealed improvement in state (Figure 3).
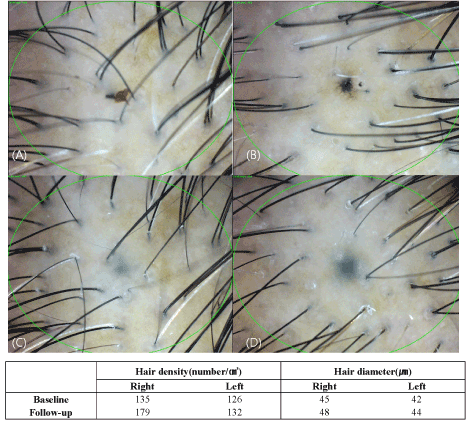
Figure 2. Evaluation of phototrichogram after treatment for 3 months (A: baseline, right side of the scalp, B: baseline, left side of the scalp, C: after 3 months, right side of the scalp, D: after 3 months, left side of the scalp).
Table 2. Mean increase in hair density and diameter after 3-month growth factor cocktail treatment with microneedling.
|
|
|
|
|
Mean increase |
Cellcurin |
p-value |
Normal saline |
p-value |
Hair density(number/cm2) |
27.1(21.4%) |
<0.0001 |
5.4(4.1%) |
0.0008 |
Hair diameter(µm) |
2.7(5.3%) |
<0.0001 |
-0.6(-0.7%) |
0.3007 |
The treatment efficacy
Split test was conducted to determine the efficacy of Cellcurin in AGA patients with microneedling. Patients’ scalp was divided into right and left sides and treated with Cellcurin on the right side and normal saline on the left side. The changes in the density and diameter of hair between the right and left sides after 3 months of treatment are shown in Table 3. Both the sides revealed augmentation in results with enhanced results on the right side as compared to the left side. The change in hair density from the baseline was 27.1 ± 10.92/cm2 on the right side and 5.4 ± 5.6/cm2 on the left side. The density of hair on the right side increased significantly as compared to that of left side with statistical significance. The change in hair diameter was 2.72 ± 1.36 µm on the right side and -0.61 ± 2.43 µm on the left side and the difference was statistically significant (p<0.05, Table 3). To investigate the effect of FGF9 on hair density and diameter, we compared the data from present study (Cellcurin; GFC with FGF9) with our previous data (SGF57; GFC without FGF9) [6], and found that hair density increased more in response to Cellcurin treatment, but no difference was observed in hair diameter (Figure 4). Side effect of the treatment involved minimal tingling sensation in some patients.
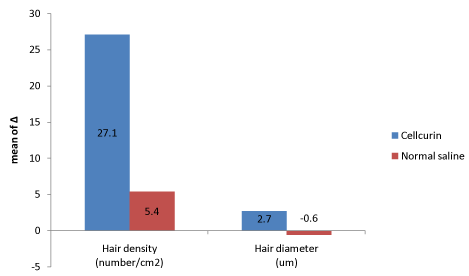
Figure 3. Efficacy comparison between Cellcurin (GFC with FGF9) and normal saline.
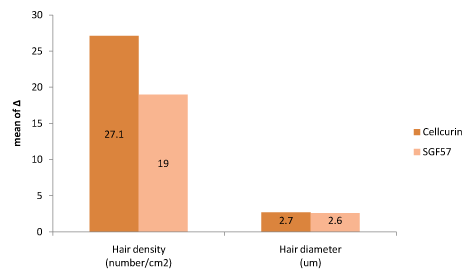
Figure 4. Efficacy comparison between Cellcurin (GFC with FGF9) and SGF57 (GFC without FGF9).
Table 3. Statistical analysis of the changes in degree of hair density and diameter between baseline and at 3 months.
|
|
|
|
|
|
|
|
Baseline |
3 months |
|
|
|
|
Mean ± SD |
Mean ± SD |
Δ |
p-value |
Hair density |
Cellcurin |
131.2 ± 21.9 |
158.3 ± 21.7 |
27.1 ± 10.92 |
p<0.0001 |
(number/ cm2) |
Normal saline |
131.6 ± 20.8 |
136.9 ± 21.8 |
5.4 ± 5.6 |
p=0.0008 |
Hair diameter |
Cellcurin |
53.3 ± 11.9 |
56.1 ± 12.0 |
2.72 ± 1.36 |
p<0.0001 |
(µm) |
Normal saline |
53.06 ± 11.9 |
52.4 ± 10.9 |
-0.61 ± 2.43 |
p=0.3007 |
We also compared our data (Cellcurin) with the previously reported clinical data by other group involving 5 % and 2 % minoxidil solution (Figure 5) [9]. The treatments with minoxidil solution were conducted for 1-year period with twice a daily topical application. Comparatively, Cellcurin was found to be ~56 % superior to 5 % minoxidil solution, which is the current dominator of the topical hair market.
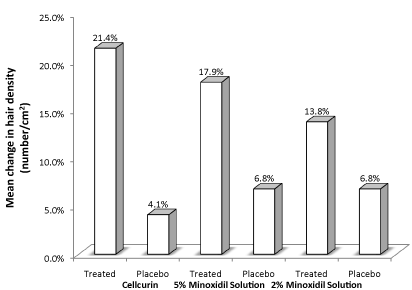
Figure 5. Comparison of clinical data of Cellcurin (GFC with FGF9) treatment and treatment with minoxidil solution.
Discussion
Hair follicle is a complex biological structure and an important functional unit for hair growth. Hair follows a specific growth cycle with three distinctive phases; anagen, catagen, and telogen, and a variety of growth factors and signaling molecules are involved during the transition of hair follicles from one phase to another [10,11]. Several growth factors such as FGF5, EGF, BDNF, and TGF-ß family have been reported as regulators of the anagen-catagen transition. Wnts and Shh are important regulators for the telogen-catagen transition. Noggin is involved in the differentiation of hair follicles [12]. Hair follicles in the anagen phase contain several growth factors such as IGF-1, EGF, FGFs, and PDGF, which bind with relevant tyrosine kinase receptors and are activated through two signaling pathways, the Ras-Raf-MEK-ERK pathway and the phosphatidylinositol-3-kinase (PI3K)-PDK1-Akt pathway, and the Ras pathway is known to regulate cell migration and proliferation, survival, differentiation, and senescence [13]. Recently, FGF9 modulates have been reported as the regenerator in hair follicle [7].
Disorders associated with hair follicles can be classified into three types, alopecia, hirsutism, and hair shaft disorders, and AGA is the most common disorder related to hair follicles [13]. AGA occurs in men and women with genetic predisposition, coupled with the presence of sufficient circulating androgens and with the more sensitive androgen receptor. The hairs in the scalp become gradually thin and eventually fall off according to pre-determined patterns [14]. The disorder typically starts when patients are in their 20s, and affects nearly 50% of adults in western countries and about 15~20% of adults in Korea [2]. Although AGA does not cause any serious direct health consequences, it can cause negative influences on the quality of life along with psychological distress and impaired social function [5].
Even though many etiologies of AGA remain to b e unknown, the major contributor is dihydrotestosterone (DHT) that is present in the hair follicles, either through the dermal papillae (DP) capillaries, or by conversion of testosterone in balding DP cells. The presence of DHT causes upregulation of IL-6, which in turn, inhibits hair shaft elongation with simultaneous suppression of matrix cell proliferation [13]. Based on these etiologies, two treatment strategies involving two most commonly used drugs are; a long-term oral administration of finasteride and a local topical application of a minoxidil solution [15].
Several cytokines, growth factors, hormones, neuropeptides, and enzymes are involved in the normal hair cycle and it is hypothesized that these substances might be involved in the pathogenesis of AGA [16]. Amongst the above-mentioned entities, growth factors are considered to play a key role in the regulation of the hair cycle, including VEGF, IGF-1, and FGFs [17]. Nowadays, safety associated with oral treatment involving finasteride and the efficacy of the topical treatment of minoxidil are questioned; henceforth, new therapeutic approaches including growth factor treatment, are being investigated [18].
In this study, we have investigated the efficacy of Cellcurin for the treatment of AGA (ranging from medium to severe state). Cellcurin contains bFGF, VEGF, IGF-1, KGF-2, SCF, and FGF9 as main ingredients, in addition to SOD and noggin peptide.
Lee et al. [5] have reported the effect of GFC without FGF9 (SGF57) on the short-term treatment of FPHL for a period of 5 weeks. In their study, the SGF57 solution was topically applied on one-half of the scalp and the other half of the scalp was treated with normal saline, both by microneedle therapy. The researchers found a significant increase in hair density in the treated half of the scalp [5]. In this study, we investigated the effect of Cellcurin on the treatment of MPHL and FPHL for a 3-month period. To enhance skin penetration of the growth factors, the topical application of Cellcurin was combined with microneedle therapy. As a result, the mean values of hair density and thickness after 3 months of treatments increased by 21.4% and 5.3%, respectively, compared with the patients’ baseline values (Table 2). Among the 18 total patients enrolled in the present study, 12 patients showed dramatic results (an increase >15%) in hair density. Gender differences were not apparent.
In general, hair research should be carried out for more than 3 months (12 weeks). The present study is of significance because of the length of the treatment period, and involvement of both MPHL and FPHL in comparison with the previous study conducted by Lee et al. [5].
Cellcurin is formulated to contain several factors, which can promote the cell cycle, the regeneration of hair cells, and protect hair follicles from apoptotic signals. It has been shown that VEGF strongly induces perifollicular vascularization, resulting in accelerated hair regrowth and increase in size of hair follicles and hair shafts [19]. Ozeki et al. [20] reported that the controlled release of growth factors such as bFGF and VEGF significantly increases the size of hair follicles in mice. IGF-1 has been shown to stimulate hair follicle growth in a dose-dependent manner and prevent hair follicles from entering into a catagen-like state [21]. KGF has been reported to protect hair follicles from the apoptotic signals induced by harmful environmental factors [22]. Noggin is an important regulator of hair cycle and acts as an inhibitor for BMP4, which suppresses the transition of telogen to anagen [23]. Noggin overexpression results in an increase in hair follicle size [24]. SCF has been demonstrated to play a significant role in skin and hair pigmentation by activating melanocytes through the c-kit pathway [25]. In AGA, large and pigmented hairs are gradually replaced by tiny and pale hairs due to the inhibition of dermal papilla SCF production by androgens. Cultured dermal papilla cells obtained from patients with AGA secrete less SCF than normal cells [26]. Oxidative stress has been suggested to play an important role in hair graying and hair loss [27]. It has been shown that antioxidants scavenge the reactive oxygen species (ROS) generated by cisplatin and protect the hair follicles from apoptosis [28]. SOD catalyzes dismutation of superoxide radicals, the most toxic ROSs, and is known to play a major role in mediating antioxidant defense in nearly all living cells exposed to oxidative stress. FGF9 has been reported as a triggering factor for Wnt expression and as hair follicle regenerator [8].
In this study, we investigated the effect of GFC including FGF9 on the treatment of MPHL and FPHL, for a period of 3 months. Both, hair density and thickness increased significantly with no side effects, except for minor irritations on the treated part of the scalp.
Limitations of this study were the relatively small number of enrolled patients and comparatively shorter study period. Eventually, studies with GFC including FGF9 need further evaluation in larger group with longer period. However, our study is the first to evaluate the efficacy of GFC including FGF9 (Cellcurin) in AGA patients in a clinical study.
Conclusion
In this study, we conf2021 Copyright OAT. All rights reservis effective for improving hair growth and thickening and the effect of GFC treatment with FGF9 was more effectual than GFC without FGF9 in hair density. Until date, there exist only few clinical studies on treatment of AGA with GFC including FGF9. Therefore, it is conjectured that this study would be helpful in providing fundamentals to further studies concentrating on effectiveness of GFC including FGF9 (Cellcurin). It is proposed that Cellcurin could be a therapeutic option for treatment of AGA.
Conflict of interest
No potential conflict of interest relevant to this article was reported.
Acknowledgements
This research was supported by PNP Biopharm, Seoul, Korea.
*This research was supported by Research Program for Clinical Professor of Myongji Hospital
*This manuscript was presented at the 17th meeting of the European Hair Research Society, Tbilisi, Gerogia, June 24-26, 2016
References
- Otberg N, Shapiro J (2011) Hair growth disorders. Fitzpatrick’s dermatology in general medicine. 8th ed. New York: MacGraw-Hill, pp.979-987.
- The Korean Dermatological Association (2014) Textbook of Dermatology. 6th ed. Medbook, pp.550-553.
- Ellis JA, Sinclair R, Harrap SB (2002) Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med 4: 1-11. [Crossref]
- Lin WH, Xiang LJ, Shi HX, Zhang J, Jiang LP, et al. (2015) Fibroblast growth factors stimulate hair growth through β-catenin and Shh expression in C57BL/6 mice. Biomed Res Int 2015: 730139. [Crossref]
- Lee YB, Eun YS, Lee JH, Cheon MS, Park YG, et al. (2013) Effects of topical application of growth factors followed by microneedle therapy in women with female pattern hair loss: a pilot study. J Dermatol 40: 81-83. [Crossref]
- Ro BI, Son HO, Chun SW, Shin HC (2016) Therapeutic effects of growth factor cocktail treatment in patients with androgenetic alopecia according to the depth of microneedle. Korean J Dermatol 54: 184-189.
- Chandrashekar B, Yepuri V2, Mysore V2 (2014) Alopecia areata-successful outcome with microneedling and triamcinolone acetonide. J Cutan Aesthet Surg 7: 63-64. [Crossref]
- Gay D, Kwon O, Zhang Z, Spata M, Plikus MV, et al. (2013) Fgf9 from dermal γδ T cells induces hair follicle neogenesis after wounding. Nat Med 19: 916-923. [Crossref]
- Lucky AW, Piacquadio DJ, Ditre CM, Dunlap F, Kantor I, et al. (2004) A randomized, placebo-controlled trial of 5% and 2% topical minoxidil solutions in the treatment of female pattern hair loss. J Am Acad Dermatol 50: 541-553. [Crossref]
- Ro YS, Seo DH, Yu DS, Lee SY, Sim WY, et al. (2014) Diseases of the skin appendages, In: The Korean Dermatological Association. Textbook of Dermatology. 6th ed. Medbook pp.550-553.
- Price VH (1999) Treatment of hair loss. N Engl J Med 341: 964-973. [Crossref]
- Alonso L, Fuchs E (2006) The hair cycle. J Cell Sci 119: 391-393. [Crossref]
- Rishikaysh P, Dev K, Diaz D, Qureshi WM4, Filip S5, et al. (2014) Signaling involved in hair follicle morphogenesis and development. Int J Mol Sci 15: 1647-1670. [Crossref]
- Ellis JA, Sinclair R, Harrap SB (2002) Androgenetic alopecia: pathogenesis and potential for therapy. Expert Rev Mol Med 4: 1-11. [Crossref]
- Kaufman KD, Olsen EA, Whiting D, Savin R, DeVillez R, et al. (1998) Finasteride in the treatment of men with androgenetic alopecia. Finasteride Male Pattern Hair Loss Study Group. J Am Acad Dermatol 39: 578-589. [Crossref]
- Panchaprateep R, Asawanonda P (2014) Insulin-like growth factor-1: roles in androgenetic alopecia. Exp Dermatol 23: 216-218. [Crossref]
- Parsley WM, Perez-Meza D (2010) Review of factors affecting the growth and survival of follicular grafts. J Cutan Aesthet Surg 3: 69-75. [Crossref]
- Chaudhari ND, Sharma YK, Dash K, Deshmukh P (2012) Role of Platelet-rich Plasma in the Management of Androgenetic Alopecia. Int J Trichology 4: 291-292. [Crossref]
- Yano K, Brown LF, Detmar M (2001) Control of hair growth and follicle size by VEGF-mediated angiogenesis. J Clin Invest 107: 409-417. [Crossref]
- Ozeki M, Tabata Y (2003) In vivo promoted growth of mice hair follicles by the controlled release of growth factors. Biomaterials 24: 2387-2394. [Crossref]
- Philpott MP, Sanders DA, Kealey T (1994) Effects of insulin and insulin-like growth factors on cultured human hair follicles: IGF-I at physiologic concentrations is an important regulator of hair follicle growth in vitro. J Invest Dermatol 102: 857-861. [Crossref]
- Braun S, Krampert M, Bodó E, Kümin A, Born-Berclaz C, et al. (2006) Keratinocyte growth factor protects epidermis and hair follicles from cell death induced by UV irradiation, chemotherapeutic or cytotoxic agents. J Cell Sci 119: 4841-4749. [Crossref]
- Zhang J, He XC, Tong WG, Johnson T, Wiedemann LM, et al. (2006) Bone morphogenetic protein signaling inhibits hair follicle anagen induction by restricting epithelial stem/progenitor cell activation and expansion. Stem Cells 24: 2826-2839. [Crossref]
- Sharov AA, Sharova TY, Mardaryev AN, Tommasi di Vignano A, Atoyan R, et al. (2006) Bone morphogenetic protein signaling regulates the size of hair follicles and modulates the expression of cell cycle-associated genes. Proc Natl Acad Sci USA 103: 18166-18171. [Crossref]
- Grichnik JM, Burch JA, Burchette J, Shea CR (1998) The SCF/KIT pathway plays a critical role in the control of normal human melanocyte homeostasis. J Invest Dermatol 111: 233-238. [Crossref]
- Randall VA, Jenner TJ, Hibberts NA, De Oliveira IO, Vafaee T (2008) Stem cell factor/c-Kit signalling in normal and androgenetic alopecia hair follicles. J Endocrinol 197: 11-23. [Crossref]
- Trüeb RM (2009) Oxidative stress in ageing of hair. Int J Trichology 1: 6-14. [Crossref]
- Luanpitpong S, Nimmannit U, Chanvorachote P, Leonard SS, Pongrakhananon V, et al. (2011) Hydroxyl radical mediates cisplatin-induced apoptosis in human hair follicle dermal papilla cells and keratinocytes through Bcl-2-dependent mechanism. Apoptosis 16: 769-782. [Crossref]





