Foci of hepatic calcification found at term and acute tubular necrosis found 48-72hrs after creating bladder outlet obstruction suggested that the model caused significant stress. We attempted to define the contribution from each step in our model to these findings. Twenty-one ewes carrying 27 60-day gestation lambs were anesthetized using nitrous oxide, oxygen and isoflourane, in 3 groups:- Anesthesia only for 60 minutes (Group A), Uterine exposure through a left flank incision for 30 minutes (Group B), Uterine exposure with each fetal lamb exposed through a hysterotomy for 12 minutes (Group C). They were delivered by cesarean section 48-72 hours later with histological changes in the heart, intestinal tract, liver and kidney compared. There were 11 lambs in Group A, 10 in Group B, and 6 in Group C. Central Hepatic Necrosis was found only in lambs euthanized using pentobarbital. There were histological changes (vacuolated degeneration in the proximal tubules) in the kidneys, in 1 Group A lamb, in no lambs in Group B and in all but 1 lamb in Group C. Fetal surgery causes vacuolated degeneration in fetal lambs’ kidneys. This may indicate significant renal stress, possibly indicating potential long-term renal damage.
fetal surgery, anesthesia, surgical stress
Over the past 15 years, we have developed and refined a series of models exploring renal and bladder development in the ovine fetus [1-15]. In some lambs delivered at term, there were small foci of calcification in the central portion of the liver, suggesting an episode of central hepatic necrosis earlier in gestation. In lambs sacrificed in the first few days after the creation of a bladder outlet obstruction, signs suggesting an episode of acute tubular necrosis (ATN) were frequently seen [6]. We decided to test each step of the surgery, short of actually creating the obstruction, to determine what effect, if any, could be attributed to the anesthetic and the exposure of the lamb. There are no reports describing how fetal anesthesia affects the development of fetal organs. We hypothesized that these studies might shed some light on the effects anesthesia might have on human fetuses undergoing open fetal surgical procedures.
After approval was obtained from the Animal Ethics Committee of Wellington School of Medicine and Health Sciences, University of Otago, Wellington Animal Ethics Committee (Wellington, New Zealand, approval number 2-11) twenty-one timed gestation ewes (60-day’s gestation) were transported from the farm 24 to 48 hours before the operation. They were examined by ultrasound to confirm the pregnancy and avoid unnecessary operations. The ewes were initially induced using intravenous thiopentone (20 mg/kg). They were then intubated and anesthesia was maintained on spontaneous respiration with a 50% nitrous oxide and 50% oxygen mixture, and 1% Isoflourane. Isoflourane was maintained at 1-2% with the level adjusted as required to provide a safe, effective anesthetic. The current approach is a modification of our original approach to the pregnant ovine uterus. The modifications include induction using intravenous (IV) Thiopentone, rather than Halothane inhalation, and routine monitoring of maternal heart rate and oxygen saturation. Blood pressure is not routinely monitored. The use of bupivacaine injected into the subcutaneous tissue and directly around the exposed segmental nerves during the incision removes the need for post-operative analgesia.
The ewes were divided into 3 groups based on their management. Group A had anesthesia only for 60 minutes. The ewes in this group were positioned on their right side as is required for the left flank incision. Group B had the uterus exposed through a left flank incision and held exposed for 30 minutes. Group C had the uterus exposed through a left flank incision. The uterus was then opened through a transverse hysterotomy with the hindquarters of each fetal lamb exposed for 12 minutes, holding their legs. These timings were based on the time taken to create our various models of obstructive uropathy in our previous 200 fetal studies from 1999-2013 [1-15]. The mean time taken to create our obstructive uropathies has been approximately 60 minutes. For these procedures, the uterus is exposed for a mean of 30 minutes, and each fetal surgical procedure involves opening the uterus and holding the caudal end of the fetus exposed for a mean of 12 minutes; this time being shorter, in general, for male fetuses and slightly longer for female fetuses. Because the lambs in Groups A & B were not exposed at all, we decided not to attempt to monitor any of the lambs. Actual monitoring of the lambs would have been technically difficult, given their small size (Table 1) and the fact that only the caudal end of the lambs in Group C were exposed.
Table 1. Comparison of the body weight, crown-to-rump length in each group.
|
|
Group A (n=11) |
|
Group B (n=10) |
|
Group C (n=6) |
Body weight (g) |
|
71.1 ± 13.5* |
|
79.8 ± 12.2* |
|
81.7 ± 10.2* |
Crown-to-rump length (cm) |
|
13.4 ± 1.0* |
|
13.9 ± 0.9* |
|
14.0 ± 0.6* |
There was no statistically significant difference between each group.
*p>0.05
After these procedures, Group C fetuses were returned to the uterus, and the hysterotomy was closed in 2 layers with 3-0 Vicryl® (Covidien Japan INC). In Groups B and C, the ewe’s abdomen was then closed with monofilament absorbable sutures (Covidien Japan INC). All incisions were closed using aseptic surgical techniques.
At 48 to 72 hours after the first procedure, the fetuses were delivered by cesarean section under general anesthesia, using the previous incision. These times were chosen in order to be able to identify any ATN or central hepatic necrosis, as there are no signs of either effect (except the occasional focus of hepatic calcification) at term. In some lambs, the fetuses were euthanized using pentobarbital injected into the umbilical cord. This was changed to sacrifice by exsanguination, with the umbilical cord being cut while the ewe was anesthetised, for the lambs for the final 10 ewes, for the reasons reported below. The crown-to –rump length and body weight of each lamb was measured. All organs were removed immediately after birth and the organs placed in formalin. Histological changes of the heart, intestinal tract, liver and kidney were compared.
Hematoxylin and eosin (HE), and Masson’s trichrome (Masson) stains and immunohistochemistry for CD10 were used for the kidneys. Masson was used for the heart. The liver and intestinal tracts were examined by HE stained sections.
We created our graded surgical stress model in 27 fetuses at 60 days of gestation. All animals survived the initial operation. Because some of the ewes carried twins, there were 11 fetuses in Group A, 10 in Group B and 6 in Group C. There was one atypical case in which two fetuses were present in same horn. Only 1 of these was recognized at the time of the initial procedure. This lamb was exposed (Group C). The 2nd lamb was found at the time of sacrifice and was included in Group B. The crown-to-rump length and body weight of the each group model is listed in Table 1.
In the lambs in which pentobarbital was injected into the umbilical cord, we noticed changes in the central area of the liver (Figure 1a) that appeared to progress during the autopsy on these lambs. Because we were concerned that this central hepatic necrosis might be a direct effect of the pentobarbital, we decided to complete the study, sacrificing the lambs by exsanguination. None of these lambs exhibited central hepatic necrosis. On histological examination, the central portions of the liver showed extensive necrosis (b). This was not found in the livers of lambs sacrificed by exsanguination.
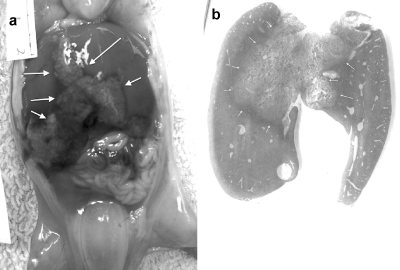
Figure 1a. Macroscopic findings of 60 days fetus liver after sacrifice with pentobarbital. The color change between the central part of the liver and the rest of the liver is outlined by the arrows.
b. The central hepatic necrosis is outlined by the arrows.
There was no statistical difference in crown-rump length or weight between the 3 groups using the unpaired Student's t test. There were no obvious histological changes in the intestinal tract each group. In the heart, ischemic changes were observed in endocardium (Figure 2), but these changes were only seen in the lambs sacrificed using pentobarbital.
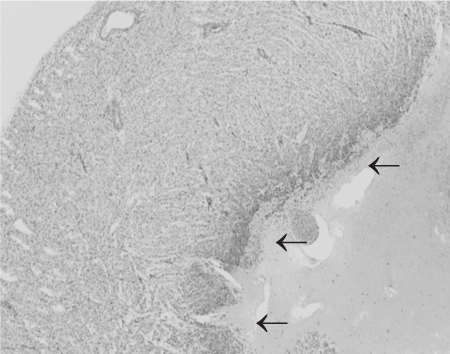
Figure 2. This is the left ventricle of the heart. There is loss of the endocardium and the subjacent myocardium. Because these features were seen only in those lambs euthanized using pentobarbitone, this is thought to be a direct toxic effect in very immature tissues
In the kidneys, we found vacuolated degeneration in the proximal tubules (Figure 3). Only one lamb had these changes in group A. In Group B, all lambs had normal histology. All lambs in Group C had some degree of vacuolated degeneration.
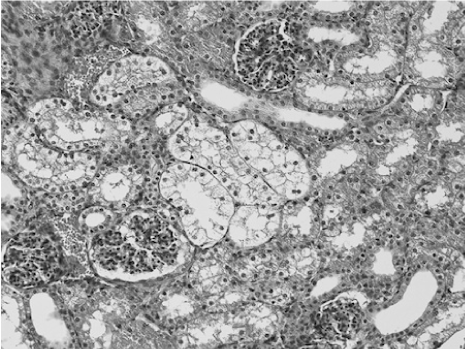
Figure 3. Typical vacuolated degeneration (clear cytoplasm) in S3 of the proximal tubules (HE)
Vacuolated degeneration of the proximal tubular epithelium was seen in segment 3 [16] of the proximal tubules (Figure 4). However, the proximal convoluted tubules were also involved in more severe cases. The vacuolated changes were divided into three groups by a scoring system according to severity evaluated by HE. For a score of 0, no vacuolar change was seen (Figure 5a). For a score of 1, there were changes seen in segment 3 (Figure 5b), and for a score of 2 the changes extended into the proximal convoluted proximal tubules (Figure 5c).
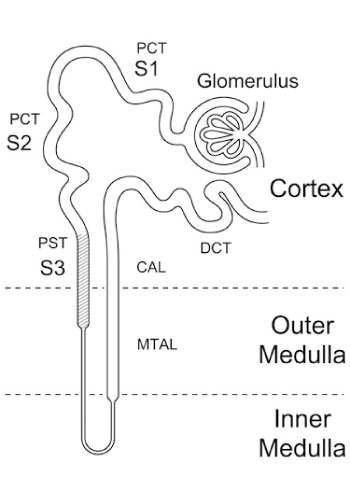
Figure 4. The tubule consists of a proximal convoluted tubule (PCT), composed of an S1, S2 and S3 segment, also known as the proximal straight tubule (PST), the loop of Henle, the medullary ascending limb (MTAL), the cortical ascending limb (CAL) and the distal convoluted tubule(DCT). (From Fujiwara et al [18].
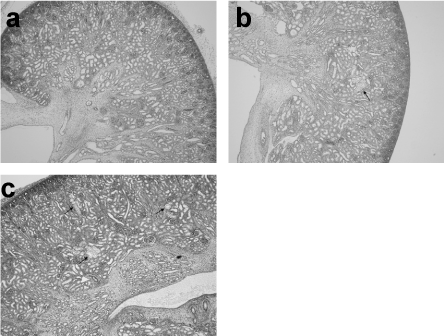
Figure 5. Scores of vacuolated degeneration of the proximal tubular epithelium. (a) No vacuolated degeneration is seen, score 0. (b) Vacuolated degeneration is seen only in S3 of the proximal tubules, score 1. (c) Vacuolated degeneration is seen in the proximal convoluted tubules as well as in S3, score 2.
By the scoring system (Table 2), in Group A there was only one case with a score of 1 and all the other lambs in this Group had a score of 0, as did all the lambs in Group B. In contrast, 3 of the lambs in Group C had a score of 1 and the other 3 had a score of 2. In comparison with the normal kidneys taken from lambs with a score of 0, dilatation of proximal tubules was seen in the kidneys of the lambs with vacuolated degeneration of the proximal tubules with a score of 2. Immunostaining using CD10, showed a significant difference in the degree of staining of the cells lining the lumen of the proximal tubules between the lambs scored as 0 and those scored as 2 (Figure 6a, b).
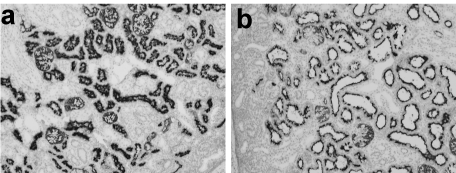
Figure 6. Immunostaining with CD10 which specifically stains the proximal tubules. Compared with a normal kidney taken from a lamb with a score of 0 (a), dilatation of proximal tubules is seen in the kidney of a lamb with vacuolated degeneration of proximal tubules, score 2 (b).
Table 2. Scores of vacuolated degeneration of proximal tubules in each group.
|
|
Group A (n=11) |
Group B (n=10) |
|
Group C (n=6) |
|
Score 0 |
10 |
10 |
|
0 |
|
Score 1 |
1 |
0 |
|
3 |
|
Score 2 |
0 |
0 |
|
3 |
The origins of fetal surgery in humans dates back to the 1960’s when several disparate groups performed open fetal surgery to perform exchange transfusions for erythroblastosis fetalis secondary to Rh incompatibility [17]. These techniques were supplanted by percutaneous intraperitoneal transfusions pioneered by Liley [18]. During the 1980s, with the development of ultrasound, there was a resurgence of interest in open fetal surgery [19,20] and the developmental pathophysiology of several potentially lethal correctable lesions was defined in animal models [21-24]. Over the ensuing 30 years, this investment in basic and clinical research has benefited an increasing number of fetal patients and has spurred continued research into the normal and abnormal development of the fetus. The clinical outcomes of antenatally-diagnosed obstructive uropathy are reported, but the long-term results are not good as had been expected [25-28]. Fetal treatment of obstructive uropathy now includes open fetal surgery, percutaneous V-A shunt placement, and fetoscopic procedures and is performed only for carefully selected patients [28-30].
2021 Copyright OAT. All rights reserv
Over the past 15 years, we have been evaluating fetal interventions for treatment of severe lower urinary tract obstruction (LUTO) and exploring renal and bladder development in a fetal lamb model [1-15], as LUTO is associated with high rates of perinatal mortality and postnatal renal impairment. Our previous studies suggest that, in the presence of a complete obstruction, there is very early dilatation of the ampulla in the nephrogenic zone [5]. This dilatation distorts the ampullae, inhibiting their normal division, effectively preventing nephrogenesis. If the obstruction is not relieved, no further nephrons are formed. In these studies on the early effects of urinary tract obstruction on glomerulogenesis, we found, within 48 hours after creating the urinary tract obstruction, that the kidneys showed extensive acute tubular necrosis (ATN). Our initial opinion was that these changes were the result of low blood flow occurring at the time of the operation to create the obstruction [5]. However, what was not clear was what contribution to these changes arose from the anesthetic and the surgical exposure required to create the model. This study was designed to address that specific question.
The typical features of human ATN on renal biopsy include vacuolation; loss of brush border in proximal tubular cells; and sloughing of tubular cells into the lumen, which leads to cast obstruction. Interstitial edema with mild to moderate leukocyte infiltration can produce widely spaced tubules. Despite the term ‘‘acute tubular necrosis,’’ frankly necrotic cells are not a common finding and often only limited histologic evidence of injury is present despite marked functional impairment [31].
In our model, histological changes are located in the so-called segment 3 area [16], which is the most sensitive area of the peripheral blood flow in the kidney. It has generally been considered that the changes of renal ischemia are first seen in the proximal tubules. In our previous obstructive uropathy model, dilatation occurred first in the proximal tubules rather than the distal tubules [5]. From these results, it would appear that the proximal tubules are very sensitive to ischemia but also to increased intraluminal pressure after obstruction. This study was designed to explore the contributions of the anesthetic alone, the exposure of the uterus and the exposure of the fetus to any renal ischemic damage. Only one lamb in the anesthesia only group (Group A) and none of the lambs in the uterine exposure group (Group B) demonstrated vacuolation. In group C, we didn’t detect two fetuses in one uterine horn at the time of operation and only one fetal lamb was exposed for 12 minutes. This lamb had a score of 1. These changes were all seen in segment 3 which is located between the renal cortex and the medulla. The other fetus which was left in the exposed uterus and was included in Group B, had no vacuolation and we scored this lamb’s kidneys as 0. We felt that it was reasonable to include this lamb in Group B as it had been exposed to exactly the same insult as the rest of the lambs in that Group. These results, we submit, strongly suggest that the histological change of vacuolation is the result of some degree of ischemia. Interestingly, sloughing of tubular cells into the tubular lumen was seen in most of the lambs in this study, which is a feature commonly seen in ATN. These changes were not located in segment 3, but were seen in the distal tubules and collecting ducts. This may imply that the fetal kidney is exquisitely sensitive to minimal hypoxia at this stage of gestation. However, ATN is completely reversible, and it is possible that even repeated insults of this nature may not be detectable at term. ATN might be more sensitive than vacuolation but this study clearly demonstrated a significant difference in the three groups using vacuolation as a marker of a more severe insult.
Immunostaining using CD10, showed a significant difference in the degree of staining cells lining the lumen of the proximal tubules between the lambs scored as 0 and those scored as 2. This might reflect a loss of the brush border in the cells of the proximal tubules, which may be a more sensitive indicator of recent proximal tubular damage. To confirm this, electron microscopy studies will be required, and these have not yet been completed. There are clear differences in the histological changes in these three groups, but more studies will be necessary to clarify the mechanism of these changes and the contribution each step in the surgical procedure makes to their development.
We postulate that the fetal kidney is very sensitive to the ischemic changes and renal vacuolation will become recognized as a reliable indicator of renal ischemia. This study suggests that in the early fetus, even a relatively mild stress can cause ischemic changes in the developing organs but the kidney would appear to be the most sensitive organ to such insults. Some of the ischemic changes are reversible after short term stresses but longer periods of ischemia or fetal stress may possibly result in permanent damage. This would suggest that long term follow-up of renal function is important in long-term survivors born with significant renal tract anomalies, and, possibly, all fetuses subjected to the effects of a maternal general anesthetic early in gestation.
Most fetal interventions are now performed using fetoscopy under sedation, rather than open fetal surgery under general anesthesia. However, open fetal surgery is still the most common approach for fetal repair of myelomeningocele [32,33] and the occasional resection of a congenital pulmonary malformation [34] or sacrococcygeal teratoma [35]. It is hoped that the graduated increase in the extent of the fetal insult reported in this study will provide some science on which to base statements about the risk of the anesthetic to the fetus in patients undergoing open fetal surgery.
- Kitagawa H, Pringle KC, Zuccolo J, Stone P, Nakada K, et al. (1999). The pathogenesis of dysplastic kidney in a urinary tract obstruction in the female fetal lamb. J Pedia Surg 34: 1678-1683. [Crossref]
- Kitagawa H, Pringle KC, Zuccollo J, Koike J, Nakada K, et al. (2000) Glomerular size in renal dysplasia secondary to obstructive uropathy: a further exploration of the fetal lamb model. J Pedia Surg 35: 1651-1655.
- Kitagawa H, Pringle KC, Zuccollo J, Koike J, Nakada K, et al. (2000) Early fetal obstructive uropathy produces Potter's syndrome in the lamb. J Pedia Surg 35: 1549-1553.
- Kitagawa H, Pringle KC, Koike J, Zuccollo J, Nakada K (2001) Different phenotypes of dysplastic kidney in obstructive uropathy in fetal lambs. J Pediatr Surg 36: 1698-1703. [Crossref]
- Kitagawa H, Pringle KC, Koike J, Zuccollo J, Seki Y, et al. (2003) Optimal timing of prenatal treatment of obstructive uropathy in the fetal lamb. J Pediatr Surg 38: 1785-1789. [Crossref]
- Kitagawa H, Pringle KC, Koike J, Zuccollo J, Sato Y, et al. (2004) The early effects of urinary tract obstruction on glomerulogenesis. J Pediatr Surg 39: 1845-1848. [Crossref]
- Sato Y, Kitagawa H, Pringle KC, Koike J, Zuccollo J, et al. (2004) Effects of early vesicostomy in obstructive uropathy on bladder development. J Pediatr Surg 39: 1849-1852. [Crossref]
- Kitagawa H, Pringle KC, Koike J, Zuccollo J, Sato Y, et al. (2005) Fetal hydrops in experimental obstructive uropathy resolves after vesicostomy formation: is this cause and effect? Pediatr Surg Int 21: 25-28. [Crossref]
- Kitagawa H, Pringle KC, Koike J, Zuccollo J, Sato Y, et al. (2006) Vesicoamniotic shunt for complete urinary tract obstruction is partially effective. J Pediatr Surg 41: 394-402.
- Kitagawa H, Pringle KC, Koike J, Nagae H, Zuccollo J, et al. (2006) Early bladder wall changes after creation of obstructive uropathy in the fetal lamb. Pediatr Surg Int 22: 875-879. [Crossref]
- Nagae H, Kitagawa H, Pringle KC, Koike J, Zuccollo J, et al. (2006) Pressure-limited vesico-amniotic shunt tube for fetal obstructive uropathy. J Pediatr Surg 41: 2086-2089. [Crossref]
- Kitagawa H, Pringle KC, Koike J, Nagae H, Zuccollo J, et al. (2007) Is a vesicoamniotic shunt intrinsically bad? Shunting a normal fetal bladder. J Pediatr Surg 42: 2002-2006. [Crossref]
- Aoba T, Kitagawa H, Pringle KC, Koike J, Nagae H, et al. (2008) Can a pressure-limited vesico-amniotic shunt tube preserve normal bladder function? J pediatr surg 43: 2250-2255.
- Kitajima K, Aoba T, Pringle KC, Seki Y, Zuccollo J, et al. (2010) Bladder development following bladder outlet obstruction in fetal lambs: optimal timing of fetal therapy. J pediatr surg 45: 2423-2430.
- Kitajima K, Aoba T, Pringle KC, Seki Y, Zuccollo J, et al. (2013) Valved shunt as a treatment for obstructive uropathy: does pressure make a difference? Pediatr surg int 29: 381-386.
- Fujiwara K., Shin M, Matsunaga H, Saita T, Larsson LI (2009) "Light-microscopic immunocytochemistry for gentamicin and its use for studying uptake of the drug in kidney". Antimicrob Agents Chemother 53: 3302-3307.
- Pringle KC (1986) Fetal surgery: it has a past, has it a future? Fetal Ther 1: 23-31. [Crossref]
- Liley AW (1963) Intrauterine transfusion of foetus in haemolytic disease. Br Med J 2: 1107-1109. [Crossref]
- Harrison MR, Golbus MS, Filly RA, Callen PW, Katz M, et al. (1982) Fetal surgery for congenital hydronephrosis. N Engl J Med 306: 591-593. [Crossref]
- Harrison MR, Filly RA, Golbus MS, Berkowitz RL, Callen PW (1982) Fetal treatment 1982". N Engl J Med 307: 1651-1652.
- Harrison MR, Ross NA, de Lorimier AA (1981) Correction of congenital diaphragmatic hernia in utero. III. Development of a successful surgical technique using abdominoplasty to avoid compromise of umbilical blood flow. J Pediatr Surg 16: 934-942. [Crossref]
- Soper RT, Pringle KC, Scofield JC (1984) Creation and repair of diaphragmatic hernia in the fetal lamb: techniques and survival. J Pediatr Surg 19: 33-40. [Crossref]
- Michejda M (1984) Intrauterine treatment of spina bifida: primate model, Zeitschrift fur Kinderchirurgie : organ der Deutschen, der Schweizerischen und der Osterreichischen Gesellschaft fur Kinderchirurgie. Surgery in infancy and childhood 39: 259-261.
- Pringle KC, Turner JW, Schofield JC, Soper RT (1984) Creation and repair of diaphragmatic hernia in the fetal lamb: lung development and morphology. J Pediatr Surg 19: 131-140. [Crossref]
- Holmes N1, Harrison MR, Baskin LS (2001) Fetal surgery for posterior urethral valves: long-term postnatal outcomes. Pediatrics 108: E7. [Crossref]
- Clark TJ, Martin WL, Divakaran TG, Whittle MJ, Kilby MD (2003) Prenatal bladder drainage in the management of fetal lower urinary tract obstruction: a systematic review and meta-analysis. Obstet Gynecol 102: 367-382. [Crossref]
- Woodhouse CR, Neild GH, Yu RN, Bauer S (2012) Adult care of children from pediatric urology. J Urol 187: 1164-1171. [Crossref]
- Morris RK, Kilby MD (2011) Long-term renal and neurodevelopmental outcome in infants with LUTO, with and without fetal intervention. Early Hum Dev 87: 607-610. [Crossref]
- Ruano R (2011) Fetal surgery for severe lower urinary tract obstruction. Prenat Diagn 31: 667-674. [Crossref]
- Morris RK, Ruano R, Kilby MD (2011) Effectiveness of fetal cystoscopy as a diagnostic and therapeutic intervention for lower urinary tract obstruction: a systematic review. Ultrasound in obstetrics & gynecology 37: 629-637.
- Lameire N (2005) The pathophysiology of acute renal failure. Crit Care Clin 21: 197-210. [Crossref]
- Adzick NS, Thom EA, Spong CY, Brock JW 3rd, Burrows PK, et al. (2011) A randomized trial of prenatal versus postnatal repair of myelomeningocele. N Engl J Med 364: 993-1004. [Crossref]
- Cohen AR, Couto J, Cummings JJ, Johnson A, Joseph G, et al. (2014) Position statement on fetal myelomeningocele repair. Am J Obst Gynecol 210: 107-111. [Crossref]
- Witlox RS, Lopriore E, Oepkes D (2011) Prenatal interventions for fetal lung lesions. Prenat Diagn 31: 628-636. [Crossref]
- Gucciardo L, Uyttebroek A, De Wever I, Renard M, Claus F, et al. (2011) Prenatal assessment and management of sacrococcygeal teratoma. Prenat Diagn 31: 678-688. [Crossref]






