Abstract
In this study, we aimed to observe the targeted biodistribution of carbon-coated pure iron core (Fe@C) magnetic nanoparticles, conjugating with Bevacizumab (BMN) in liver cancer xenografts. We inoculated SSMC7721 cells subcutaneously into the posterior flank. All mice were randomly divided into five groups (n=8 for each group). Group 1 was the control group, injected normal saline through the tail vein. Mice in group 2 and 3 were injected epirubicin and bevacizumab respectively through the tail vein. Group 4 and group 5 were injected with BMN and Fe@C without Bevacizumab respectively. Both group 4 and group 5 were subjected to a magnetic field at 3000 gauss bulit on the surface of tumor for 30 min once a day. All mice were euthanized after treatment of 7 days. The tumors were resected, and were stained with hematoxylin and eosin. Following staining with HE, tumor specimens from all groups were examined for apoptosis by a TUNEL assay. The microvessel density (MVD) was evaluated by the method of immunohistochemistry. We found the complete tumor suppression was observed in group 2 and 4. The apoptotic indexs in groups 2 and 4 were 73.3% ± 8% and 63.1% ± 7%, respectively. They was no different significantly with each other (P>0.01). The MVD decreased to 21.75 ± 3.96 in group 4 and 40.25 ± 3.85 in group 3. There was significant difference between group 4 and other groups. Our study indicated the possibility of BMN preparation and treatment for tumor. BMN could play the important role in tumoricidal effect.
Key words
magnetic nanoparticles, bevacizumab, hepatoma
Introduction
Hepatoma is one of the most common tumors worldwide, and hepatocellular carcinoma (HCC) is the most common type of liver cancer. Hepatoma causes more than 700,000 deaths worldwide per year. Liver cancer doesn't respond well to conventional chemotherapy and is often diagnosed too late for surgery to be operated. Many patients will die within a year of diagnosis [1,2].
Chemotherapy plays an important role in treating the hepatocellular careinoma and eliminating the tiny careinoma focus. But both the systemchemotherapy and transcatheter hepatic arterial chemotherapy cannot achieve the ideal effect without producing serious side effects. Magnetic drug nanoparticles are composed of chemotherapeutic agent, superparamagnetic materials and possesses the long circulating ability.
When it is injected into body, it can accumulate at the target region under the magnetic field, and then drug is released smoothly to kill the tumor cells, avoiding systematic toxicity [3-5]. In this study, we established a carbon-coated pure iron core (Fe@C) magnetic nanoparticles, conjugating with Bevacizumab (BMN). The effects of targeted treatment of BMN in nude mice with the transplanted human hepatoma cell line SSMC7721 were observed.
Materials and methods
Preparation of BMN
One hundred milligram sodium alginate (Sigma, USA) and 100 mg Fe@C (Junye Nanomaterials Co., Ltd., China) were dissolved in 6 ml purified water, and mixed by ultrasound for 15 min.The mixture was put in 90 ml dioctyl sodium sulfosuccinate(AOT)/heptane oil phase at 60°C drop by drop. Then 4ml CaCl2 solution was added to facilitate magnetic separation, the microemulsion rinsed with acetone, and distilled water, then centrifuged and separated. One milligram of magnetic nanoparticles was washed with phosphate buffer solution, then mixed with 5mg carbodiimide (Sigma, USA) at room temperature for 15 min. Fifty milligrams of 6-aminocaproic acid was added and stirred at room temperature for 3 h, and followed by addition of 500 μL (40 μg/mL) Bevacizumab (BD Pharmingen, USA) and stirred gently for 6 h. Samples were blocked with 0.2 M glycine solution containing 1 mL of 0.2% bovine serum albumin (Gibco, USA) and stored at 4°C after magnetic separation. The morphology was investigated under atomic force microscopy and light microscope (Nike, TE2000-U, Japan).
Cell culture
The SSMC7721 cells were maintained at 37°C in RPMI 1640 medium (Gibco Invitrogen, Carlsbad, CA) supplemented with 10% heat-inactivated fetal bovine serum, 0.2% sodium bicarbonate, 2 mM glutamine, 100U/mL penicillin, and 100 mg/mL streptomycin, in humidified air with 5% CO2. The cells used in the experiment were in log phase with 23.5 h doubling time (the period of time required for a quantity to double in size or value). Cell viability before treatment was always over 98%. The SSMC7721 cell line was supplied by the China Center for Type Culture Collection (Wuhan University, China).
Development of xenografts
All experiments were carried out in accordance with protocols approved by the local experimental animal ethics committee. Forty BALB/c nude mice aged 4 weeks and weigh-ing approximately 20 g were obtained from Hubei Center for Diseases Control and Prevention (Wuhan, China). All mice were injected with cell suspensions, consisting of approximately 5 × 106 cells in 0.1 mL of phosphate-buffered saline, subcutaneously into the posterior flank. Seven days after tumor inoculation, the tumor diameter was about 8 × 5 mm and the mice were given treatment. All mice were randomly divided into five groups (n=8 for each group). Group 1 was the control group, injected normal saline through the tail vein. Mice in group 2 and 3 were injected epirubicin (0.001 mg) and bevacizumab (0.001 mg) respectively through the tail vein. Group 4 and group 5 were injected with BMN (0.001 mg) and Fe@C (0.001 mg) without Bevacizumab respectively. Both group 4 and group 5 were subjected to a magnetic field at 3000 gauss bulit on the surface of tumor for 30 min once a day. The magnetic field was supplied by 2 cm × 2 cm NdFeB (Fuyong, Co.,Ltd., Shenzhen) which could provide the permanent magnetic strength of 3000Gs. Tumor volumes (V) were measured everyday and calculated using the formula: V = π/6 × larger diameter × (smaller diameter) 2.
Histologic examination
All mice were euthanized after treatment of 14 days. The tumors were resected, fixed with 10% formalin solution, overnight and embedded in paraffin. Tissue sections (4 μm) were stained with hematoxylin and eosin. Following staining with hematoxylin and eosin, tumor specimens from all groups were examined for apoptosis by a TUNEL assay (Promega, USA), in accordance with the manufacturer’s instructions. Apoptosis was evaluated using the apoptotic index. Briefly, 500 cells were counted in each specimen, and the apoptosis index was defined as: Apoptotic index (%) = 100 × apoptotic cells/total cells. We obtained the mean of data.
Determination of MVD
MVD was evaluated by the method of immunohistochemistry and used the polyclonal antibody of rabbit anti-human factor VIII related antigen (1:100; Santa Cruz Biotechnology, Santa Cruz, CA). Stained sections were examined by light microscopy and microangiography was performed. Intratumoral MVD was recorded by counting the mean of FVIII-positive vessels in the most vascularized area in 3× 200 fields. Blood vessels with a lumen diameter exceeding approximately eight RBCs were excluded.
Statistical analysis
A statistical evaluation was performed using the Statistical Program for Social Sciences for Windows (SPSS, version 13.0). All experimental results and measurements were expressed as means ± standard deviation (SD). Differences between groups were examined for statistical significance using the Bivariate method and Student’s t test. Values of P<0.05 were considered statistically significant.
Results
Morphology and particle size of BMN
BMN were examined using an AJ-III Atomic Force Microscope (AJ Nano-Science Development Co. Ltd., Shanghai, China) in tapping mode, and showed good dispersion. Nanoparticles size was 51-90 nm with an average diameter of 63 nm (Figure 1). Protein concentration on the nanoparticles was measured by the Bradford method. Immunofluorescence staining and flow cytometry showed 95.7% of Fe@C contained bound bevacizumab.
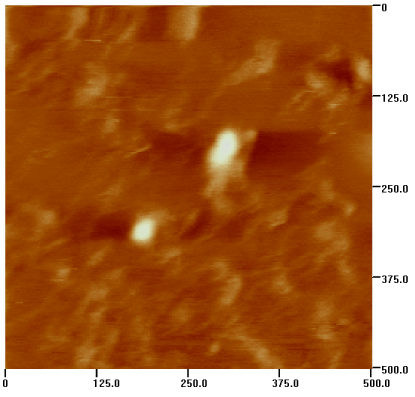
Figure 1. The picture of BMN by Atomic Force Microscope
Antitumor effects of BMN
The time course of tumor growth in each group is shown in Figure 2. Tumor volumes in group 1 and group 5 were always the biggest, then the group 3,4 and 2 sequently. From the volumes of resected tumors, we could find that group 2 has no significant difference with group 4, and that group 1 has no significant difference with group 3 or 5, either (P>0.05). The difference between group 4 and 1 or 3 and the difference between group1 and 2 were significant, respectively (P<0.05). Thus, the complete tumor suppression was observed in group 2 and 4. Histologic findings Similar to the result of tumor volumes, HE stains showed that there were most dead tumor cells in group 2,then the group 4,3 ,1and 5 sequently.
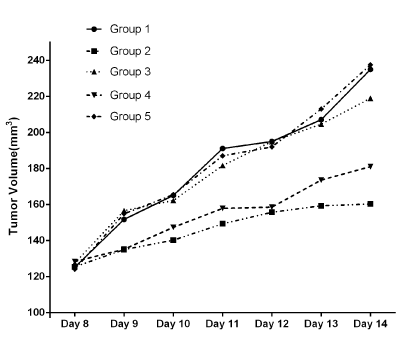
Figure 2. The time course of tumor growth in each group. The complete tumor suppression was observed in group 2 and 4
TUNEL method revealed the apoptotic index of tumor cells. Few tumor cells were positive for TUNEL staining in groups 1(Table 1). The apoptotic indexs in groups 2 and 4 were 73.3% ± 8% and 63.1% ± 7%, respectively. There are no significant difference with each other (P>0.05). Further, the apoptotic index in group 3 was 27% ± 5%. There was significant difference between groups 4 and 3 (P<0.05). These findings suggested that BMN could play the same role as epirubicin in tumoricidal effect. Bevacizumab alone had little influence to hepatoma.
| |
Group 1 |
Group 2 |
Group 3 |
Group 4 |
Group 5 |
|
| |
MVD |
56.25 ± 11.59 |
33.25 ± 3.96 |
40.25 ± 3.85 |
21.75 ± 3.96* |
57.63 ± 10.22 |
|
| |
AI |
0.119 ± 0.03 |
0.733 ± 0.08 |
0.27 ± 0.05 |
0.631 ± 0.07▲ |
0.122 ± 0.04 |
|
| |
Table 1. Results of MVD and AI
Compared with other groups :*P<0.05.
Compared with group 1 and 3:▲P<0.05.
Intratumoral MVD Observed Through Immunohistochemistry
Any yellow stained endothelial cell or endothelial cell cluster clearly separating from adjacent microvessels, tumor cells, and other connective tissue elements were considered as single microvessels. Vessel lumens were not necessary for a structure to be defined as a microvessel, and red cells were not used to define a vessel lumen (Figure 3). Table 1 showed the changes in MVD of the tumors in every group. The MVD of control tumors (group 1) was 56.25 ± 11.59, which was similar to that of group 5(P>0.05). Due to the vessel depression introduced by bevacizumab, the MVD decreased to 21.75 ± 3.96 in group 4 and 40.25 ± 3.85 in group 3. There are significant differences between group 4 with other groups. Although the group 3 had the lower MVD, the difference is not significant with group 1.
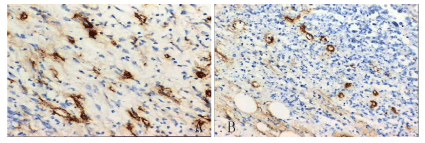
Figure 3. The MVD in group 4(A) and group 3(B). Due to the targeted biodistribution of magnetic anoparticles , Group 4 have the lowest MVD
Discussion
Epirubicin is an anthracycline drug used for chemotherapy. Similarly to other anthracyclines, epirubicin acts by intercalating DNA strands. Intercalation results with complex formation which inhibits DNA and RNA synthesis. It also triggers DNA cleavage by topoisomerase II, resulting with the mechanisms that lead to cell death. Binding to cell membranes and plasma proteins may be involved in the compound's cytotoxic effects. Epirubicin also generates free radicals that cause cell and DNA damage. Epirubicin was primarily applied against breast cancer, gastric cancer, lung cancer and liver cancer. Its main side-effects include bone marrow dysfunction, hepatic impairment and renal impairment [6,7].
Bevacizumab is a humanized monoclonal antibody that introduce angiogenesis inhibition by inhibiting the activation of vascular endothelial growth factor A (VEGF-A). VEGF-A is a chemical signal that stimulates angiogenesis in a variety of diseases, especially in cancers. Bevacizumab was the first clinically available angiogenesis inhibitor [8]. It has been approved of being applied in certain lung cancers, renal cancers, ovarian cancers, breast cancers and so on. The overwhelming data of trials for usage of bevacizumab with other medicine demonstrated its efficacy and safety, which continually extended survival for the patients. The main side effects were hypertension and heightened risk of bleeding [9]. Such drawbacks could be overcome in part by developing a carrier to enhance the concentration of the drug specifically at the tumor site. Nanotechnology, specially magnetic drug nanoparticle, holded the promise of novel and more effective treatments for tumors [10]. By far the most activity had been focused on Fe-based nanoparticles of the three elements ferromagnetic under physiological conditions, likely due to its superior magnetic susceptibility, as well as the large natural reservoir of iron in the body, suggesting the comparative absence of toxicity of this element [11]. In this study, we prepared the BMN and the efficacies of BMN were evaluated in nude mice with tumors. After 7 days, tumor growth was only slightly inhibited by bevacizumab alone. In contrast, tumors shrank markedly by the treatment of BMN with external magnetic field. Both tumor volumes and MVD in group 4 were minimum in this study. The increased efficiency could have resulted in part from the increased stability of BMN. Moreover, the BMN presumably was guided to the tumoral region by application of the external magnetic field, enhancing the local concentration of the drug. The surficial chemistry of BMN that allowed sufficient loading of therapeutics that could be deposited at the tumor site with high dose contributed to its effectiveness at the same time.
MVD is currently considered the gold standard for histological assessment of the degree of angiogenesis within a tumor. It is an independent predictor for the growth, metastasis and prognosis of tumor. Increasing evidence suggested that there was a clear relationship between the MVD and HCC prognosis [12,13]. The low MVD in group 4 confirmed that BMN could retain in tumor tissue and inhibit vessel growth by application of the external magnetic field.
The internalized particles containing Fe ensured these diffraction patterns of the selected area. Interestingly, the effect of BMN with magnetic field approached that of epirubicin in our study. But the treatment of bevacizumab alone showed little effect to tumor. It proved that the application of the external magnetic field enhanced BMN effects.
Moreover, the cytotoxi2021 Copyright OAT. All rights reservs the therapeutic prodrug would not exert its function until reaching the target environment, which helped minimizing harm to normal tissue [14,15]. As we know, chemotherapeutics drugs have many serious side-effects. If BMN nearly has the same therapeutic effect as epirubicin, we assumed that increasing BMN should decrease the dose of chemotherapeutics drug, which could avoid the side-effects maximumly.
In conclusion, our preliminary study indicated the possibility of BMN preparation and treatment for tumor. The magnetic delivery of BMN provided more efficient tumor suppression, concurrently reducing the likelihood of adverse systemic effects during chemotherapy. This was a promising strategy to transport drugs by magnetic targeting, and could enhance drug concentrations more efficiently. In order to get more proof, we looked forward further synthesized BMN in conjunction with chemotherapeutics drug in the future researches.
References
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, et al. (2011) Global cancer statistics. CA Cancer J Clin 61: 69-90. [Crossref]
- Ide T, Miyoshi A, Kitahara K, Noshiro H (2013) Prediction of postoperative complications in elderly patients with hepatocellular carcinoma. J Surg Res 185: 614-619. [Crossref]
- Klostergaard J, Seeney CE (2012) Magnetic nanovectors for drug delivery. Nanomedicine 8 Suppl 1: S37-50. [Crossref]
- Ruan J, Ji J, Song H, Qian Q, Wang K, et al. (2012) Fluorescent magnetic nanoparticle-labeled mesenchymal stem cells for targeted imaging and hyperthermia therapy of in vivo gastric cancer. Nanoscale Res Lett 7: 309. [Crossref]
- Li FR, Li Q, Zhou HX, Qi H, Deng CY (2013) Detection of circulating tumor cells in breast cancer with a refined immunomagnetic nanoparticle enriched assay and nested-RT-PCR. Nanomedicine 9: 1106-1113. [Crossref]
- Chang W, Hsieh B, Cheng H, Lee K, Chang KL et al. (2014) Progesterone augments epirubicin-induced apoptosis in HA22T/VGH cells by increasing oxidative stress and upregulating Fas/FasL. J Surg Res 188: 432-441. [Crossref]
- Rafiee E, Eavani S (2014) pH-responsive controlled release of epirubicin from Fe@Si-PW hybrid nanoparticles. Mater Sci Eng C Mater Biol Appl 39: 340-343. [Crossref]
- Liang HL, Hu AP, Li SL, Liu JY (2014) Combining bevacizumab and panitumumab with irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as second-line treatment in patients with metastatic colorectal cancer. Med Oncol 31: 976. [Crossref]
- Deng T, Zhang L, Liu XJ, Xu JM, Bai YX, et al. (2013) Bevacizumab plus irinotecan, 5-fluorouracil, and leucovorin (FOLFIRI) as the second-line therapy for patients with metastatic colorectal cancer, a multicenter study. Med Oncol 30: 752. [Crossref]
- Hua MY, Liu HL, Yang HW, Chen PY, Tsai RY, et al. (2011) The effectiveness of a magnetic nanoparticle-based delivery system for BCNU in the treatment of gliomas. Biomaterials 32: 516-527. [Crossref]
- Zhu MT, Wang Y, Feng WY, Wang B, Wang M, et al. (2010) Oxidative stress and apoptosis induced by iron oxide nanoparticles in cultured human umbilical endothelial cells. J Nanosci Nanotechnol 10: 8584-8590. [Crossref]
- Li K, Shen SQ, Xiong CL (2008) Microvessel damage may play an important role in tumoricidal effect for murine h(22) hepatoma cells with hyperthermia in vivo. J Surg Res 145: 97-104. [Crossref]
- Shen SQ, Li K, Zhu N, Nakao A (2008) Expression and clinical significance of NET-1 and PCNA in hepatocellular carcinoma. Med Oncol 25: 341-345. [Crossref]
- Chen PY, Liu HL, Hua MY, Yang HW, Huang CY, et al. (2010) Novel magnetic/ultrasound focusing system enhances nanoparticle drug delivery for glioma treatment. Neuro Oncol 12: 1050-1060. [Crossref]
- Fan C, Gao W, Chen Z, Fan H, Li M, et al. (2011) Tumor selectivity of stealth multi-functionalized superparamagnetic iron oxide nanoparticles. Int J Pharm 404: 180-190. [Crossref]



