Abstract
Subungual melanoma is a rare variant of acral melanoma, accounting for only 1–2% of cutaneous melanoma in Caucasians. Melanoma associated-epidermal/epithelial hyperplasia has been reported in association with both cutaneous melanoma and subungual melanoma. Two patterns of epithelial hyperplasia have been described in subungual melanoma, namely: proliferation of the epithelium along with elongated rete ridges in the nail bed, and longitudinal elongation of basal cells arranged compactly in the nail matrix. However, subungual melanoma developing in association with an exuberant epithelial proliferation of the nail bed presenting as numerous subungual epidermoid microcysts and solid islands of bland squamous epithelium, has never been fully characterized before. This peculiar association that we consider to be site specific, can be a potential pitfall for interpretation and diagnosis.
Key words
subungual melanoma, nail bed melanoma, nail apparatus melanoma, Hutchinson’s sign, longitudinal nail splitting, epithelial hyperplasia, subungual epidermoid inclusions
Introduction
Melanoma in general and acral melanoma, including subungual melanoma in particular, may present with epithelial hyperplasia, ranging from slightly acanthotic epithelium to pseudoepitheliomatous hyperplasia [1,2]. However, development of subungual melanoma in association with subungual epidermoid inclusions has never been fully documented before. After a careful Medline/PubMed, Eselvier ScienceDirect, Cochrane Library, and Web of Knoledge-Thomson Reuters Database search, the authors were unable to find a well-documented report of a similar finding. Only two very brief observations unaccompanied by photographic documentation of this association were found by the authors in two articles published by Fanti and Tosti [3] in 1989 in Dermatologica and by Pierrin in 2013 in The American Journal of Dermatopathology, respectively [4].
We report a very interesting case of subungual melanoma that developed along with numerous epidermoid inclusions in the nail bed dermis. Monodactylic longitudinal erythronychia with longitudinal splitting and distal onycholysis was the only clinical presentation for almost a decade, until Hutchinson’s sign appeared.
Case report
We received in consultation two skin biopsies representing periungual tegument of the left big toe of a 64-year-old woman with a clinical suspicion of acral melanoma (Figure 1). One biopsy was taken from the proximal nail fold and adjacent tegument and the other from the hyponichium of the respective toe. Both biopsies showed melanoma in situ, characterized by poorly circumscribed and asymmetric melanocytic proliferations composed of atypical melanocytes disposed exclusively at the level of the epidermis (Figure 2A). Areas with solitary non-equidistant junctional melanocytes alternated with areas of poorly formed nests displaying extreme confluence (Figure 2B). Pagetoid spread of solitary melanocytes involving all the epidermal layers was a prominent feature in both biopsies (Figure 2C). Melanocytes showed remarkable pleomorphism, being medium and large sized with hyperchromatic and irregularly contoured nuclei and intensely pigmented cytoplasm. Towards the proximal nail fold, the melanocytic proliferation extended in a lentiginous fashion along an area of pseudoepitheliomatous hyperplasia with keratin cyst formation (Figure 2C). The atypical melanocytic proliferation extended also to the epithelium of the ventral part of the proximal nail fold. Of note, the epithelium of the proximal part of the nail matrix present in the biopsy was not involved by the atypical melanocytic proliferation (Figure 2D). The exclusive confinement of melanoma to the epidermis was emphasized by the immunohistochemical stains Melan A and CD117 (Figure 2E and 2F). At this point we requested to see the clinical picture for the integration of histological findings with the clinical data. The clinical picture showed a large, poorly circumscribed, and irregularly pigmented melanocytic lesion involving the proximal nail fold and the adjacent tegument, and the distal part of the nail bed and hyponychium of the left big toe. Interestingly, the nail plate was only slightly pigmented (no true melanonychia). However, the longitudinal splitting of the nail plate with distal onycholysis pointed to the fact that the melanocytic lesion must have involved the entire nail apparatus. Upon further questioning, the patient detailed that the nail plate changes started about 10 years ago and that these changes developed after a serious toe injury. The patient ignored these changes until the periungual pigmentation started to develop, about 2 years prior to seeking medical advice. Hence, clinical-pathological correlation established that both biopsies belonged to the same lesion namely, a subungual melanoma with periungual extension (Hutchinson’s sign). A complete surgical removal of the nail apparatus was recommended. One month after the initial biopsy the patient underwent amputation at the distal interphalangeal level. The specimen was longitudinally cut in sagittal plane in multiple sections. Importantly, histological changes were restricted to the sections cut at the level of the longitudinal nail splitting. At this level, histology revealed a persistent atypical melanocytic proliferation involving both the nail bed epithelium and nail bed dermis (Figure 3A and 3B). One striking feature was the presence in the superficial and mid dermis of numerous roundish epithelial structures, some of them with central keratinization. These epithelial structures were composed of well differentiated epithelium with both onycholemmal/trichilemmal and infundibular keratinization, representing subungual epidermoid inclusions (Figure 3C and 3D). Melanocytes were disposed both as solitary units and as large confluent nests at the junction of the remnant nail bed epithelium and dermis and around the subungual epidermoid inclusions. The melanocytes were morphologically similar to the ones described in the previous biopsies. Of note, the atypical melanocytic proliferation did not extend at the level of the nail matrix epithelium (Figure 3E and 3F). Our final diagnosis was primary nail bed melanoma associated with subungual epidermoid inclusions with extension to the proximal nail fold (Hutchinson’s sign), Breslow thickness 1.4mm, pT3a (AJCC 2009).
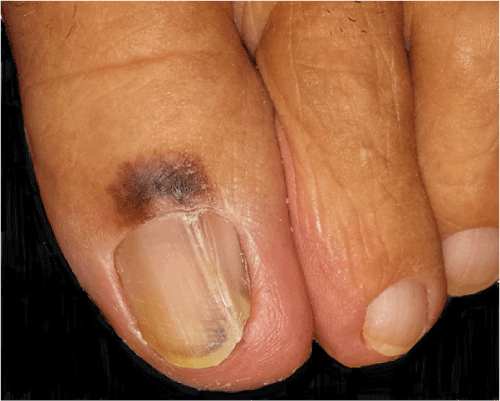
Figure 1. Nail apparatus melanoma: large, poorly circumscribed, and irregularly pigmented melanocytic lesion involving proximal nail fold and adjacent tegument (Hutchinson’s sign), distal part of the nail bed and hyponychium and nail plate of the left big toe with longitudinal splitting and distal onycholysis
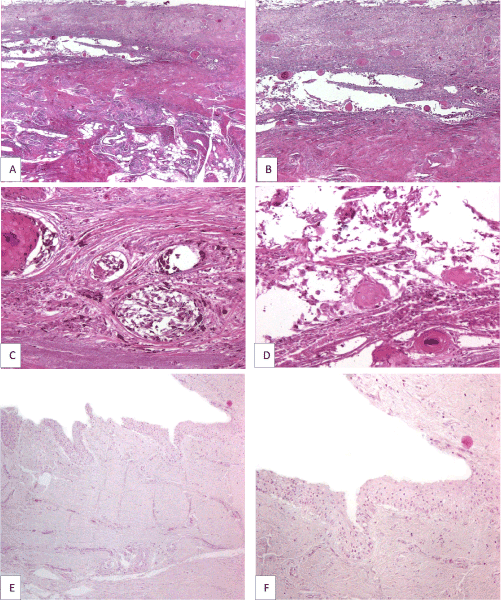
Figure 2. Melanoma in situ of the proximal nail fold and adjacent tegument: poorly circumscribed and asymmetric melanocytic proliferation composed of atypical melanocytes disposed exclusively at the level of the epidermis (A); areas with solitary inequidistant junctional melanocytes alternate with areas of poorly formed nests displaying extreme confluence (B); atypical melanocytic proliferation growing along an area of pseudoepitheliomatous hyperplasia with keratin cyst formation towards the proximal nail fold (C); uninvolved epithelium of the proximal part of nail matrix (D and F); exclusive confinement of melanoma to the epidermis emphasized by immunohistochemical stain CD117 (E and F).
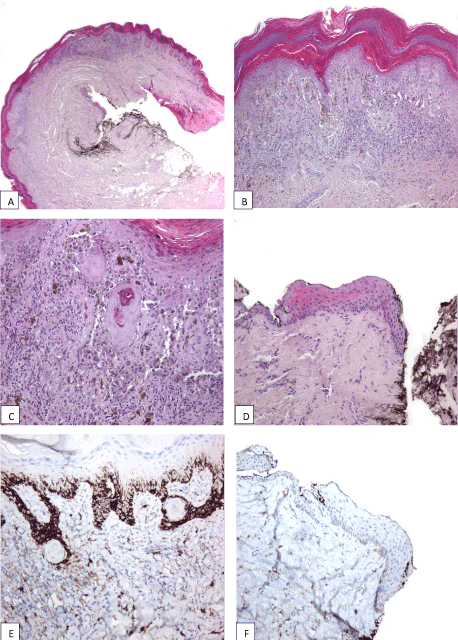
Figure 3. Longitudinally cut section through the entire midline portion of the nail bed and nail matrix, corresponding to the longitudinal nail plate splitting, showing invasive nail bed melanoma in association with subungual epidermoid inclusions (A); persistent atypical melanocytic proliferation and numerous roundish epithelial structures, some of them with central keratinization, in the superficial and mid dermis of the nail bed (B); melanocytes are disposed mainly as solitary units and large confluent nests at the junction between solid squamous islands and adjacent dermis but also away from the subungual epidermoid inclusions (C); epithelial structures are composed of well differentiated epithelium with both onycholemmal/trichilemmal and infundibular keratinization, representing subungual epidermoid inclusions (D); no involvement of nail matrix epithelium by the atypical melanocytic proliferation (E and F).
Discussion
Subungual melanoma is a rare variant of acral melanoma, accounting for only 1–2% of cutaneous melanoma in Caucasians [5,6]. It affects females more than males, usually in their 6th to 7th decades of life [7]. Exceptionally it can present in children [8]. Thumbs are more frequently involved than great toes, but it can develop in any finger [9]. Most commonly subungual melanoma arises from the nail matrix and produces a pigmented longitudinal nail band [10]. However, it may arise from the epidermis surrounding the nail, and rarely from the nail bed epithelium [11-14].
When melanoma extends from the nail matrix to the surrounding epidermis, it produces a very characteristic pigmentation of the nail fold and/or hyponychium named Hutchinson’s sign that is the hallmark of this condition [15]. In these cases, melanoma is often excised at an early stage. On the contrary, nail bed melanoma is usually hypopigmented. It can present as longitudinal erythronychia, longitudinal ridging, monodactylic longitudinal splitting or distal onycholysis mimicking a whole range of infectious or inflammatory nail diseases. Hence, it is diagnosed in a more advanced stage [12,16,17]. Frequently, the patients recall a history of trauma raising the possibility that injury may play an etiologic role. The trauma can precede the onset of disease by months to years [18,19]. In our case a traumatic event prior to the appearance of the nail plate changes was also recollected by the patient.
As is the case with other melanomas, subungual melanomas have an initial phase of intraepithelial growth. Very early lesions may show only a slightly increased number of scattered mildly atypical melanocytes disposed as solitary units at the dermal-epithelial junction with some degree of pagetoid spread. In more advanced stages of intraepithelial growth, more melanocytes with greater atypia are disposed either in linear arrangement at the dermal-epithelial junction, in a band-like proliferation, or as irregular confluent nests [20]. More obvious pagetoid extension is noticed in these cases. The pattern of dermal infiltration is different in various areas of the nail unit. Dermal invasion of the nail bed by the atypical melanocytes appears earlier than dermal invasion of the nail matrix [2,21]. As such, in nail apparatus melanoma biopsies, areas of melanoma in situ in the nail matrix can be seen adjacent to areas of obvious invasive melanoma at the level of the nail bed dermis. This was also evident in our case. The fact that neither the initial proximal nail fold biopsy nor the amputation specimen revealed atypical melanocytic proliferation in the matrix epithelium (both proximal and distal) strongly favors the nail bed as the origin of this peculiar subungual melanoma. Histologically, the heavy involvement of the nail bed and the periungual skin by the melanoma contrasts sharply with the absence of an atypical melanocytic proliferation in the nail matrix, even a very subtle one. It is unclear why this melanoma, which initially began as nail plate dystrophy, evolved without melanonychia or periungual pigmentation for a long time (almost a decade) and finally developed Hutchinson’s sign in the absence still of nail plate pigmentation. Clinical-pathologic correlation suggests the following scenario: melanoma started either in the matrix or nail bed epithelium (more likely) and then developed entirely at the level of the nail bed for a long period of time. Hence, the absence of melanonychia. From the nail bed, melanoma extended over the matrix into the nail fold with the appearance of Hutchinson pigmentation. One explanation for Hutchinson’s sign appearance in the absence of concomitant melanonychia could be the field effect phenomenon with increased lateral mobility of the neoplastic melanocytes due to Kit pathway activation [22-24]. In our case, strong c-kit expression by the melanoma cells supports this theory.
Measuring Breslow thickness in nail apparatus melanoma may pose great challenges due to the unique anatomy of this particular site, absence of the granular layer in nail matrix and nail bed epithelium, and the presence of the frequently associated epithelial hyperplasia [2,12,21]. Our case illustrates full well these difficulties. First of all, during the surgical processing of the specimen, the nail plate spontaneously avulsed and was not delivered to the laboratory. Hence, the underlying nail unit epithelium that accompanies the nail plate got detached and severely damaged also. However, careful scrutiny of the still attached viable epithelium revealed melanoma and was used as the highest point to measure the Breslow thickness. Second, the presence of the detached subungual epithelial islands with microcyst formation used as a template by the melanoma to grow deep into the nail bed dermis may seemingly overestimate the depth of invasion. Most of the atypical melanocytic proliferation was disposed as single units and confluent nests at the junction between the epithelium of those solid squamous islands and the adjacent dermis. However, some of the big confluent melanocytic nests seemed to be away from the keratinocytic nests. As such, that was the area used as the deepest front of invasion for measuring purposes.
Melanoma associated-epidermal/epithelial hyperplasia has been reported in association with both cutaneous melanoma and subungual melanoma [1,2]. Two patterns of epithelial hyperplasia have been described in subungual melanoma, namely: proliferation of the epithelium along with elongated rete ridges in the nail bed, and longitudinal elongation of basal cells arranged compactly in the nail matrix [2]. We also observed this peculiar association in our case. However, unlike the small focus of epidermal hyperplasia observed in the periungual skin biopsy, the epithelial proliferation associated with the nail bed melanoma was more exuberant, presenting as numerous subungual epidermoid microcysts and solid islands of bland squamous epithelium.
Subungual epidermoid inclusions were first described by Samman in an article published in 1959 in The British Journal of Dermatology [25]. He suggested trauma as a possible cause for these “keratin cysts arising from the nail bed”. Since then, this condition was reported by several authors under different names, such as: subungual epidermoid inclusions [26,27], subungual epidermoid cysts [28], subungual dermoid inclusions [3,29], subungual onycholemmal cysts [30], subungual calcified inclusions [31], follicular microcysts of the nail bed and microcystic nail bed hamartoma [4]. The largest series were published by Lewin [27] in 1969 and by Fanti and Tosti [3] in 1989. Lewin reported cases of subungual epidermoid inclusions associated with both clubbing and normal nails without evidence of trauma [27]. In Lewin’s opinion, subungual epidermoid inclusions associated with clubbing develop from nail bed epithelium sequestration into the dermis, the process being triggered by fibroblast proliferation. He also suggested proliferation of the tips of the nail bed rete ridges as the mechanism by which these inclusions appear in clinically normal nails [27,31]. In contrast, clubbing was not a clinical feature of subungual epidermoid inclusions in the series of 8 cases reported by Fanti and Tosti [3]. Instead, thickening and shortening of the nail plate associated with marked subungual hyperkeratosis was the main clinical presentation. Onycholysis [3,28,31], particularly in the midline nail plate, discoloration, thickening of the lateral plate forming a central cavitation [31], longitudinal and transverse ridging [32], nail bed ulceration, anonychia,3 and nail bed pigmentation [4,31], are other clinical presentations. Patients affected by this condition are usually females and males in their 5th-6th decades of life. However, a case of subungual epidermoid inclusions was reported in a 11-year-old girl [32].
Even though no initiating event could be revealed in most of the cases published to date, trauma was found as a trigger factor in half of the cases reported by Fanti and Tosti [3], as suggested in the original description of this entity [25]. In our case, the patient also recalled a trauma of the big toe several years before the appearance of the nail plate changes. In most of the cases published so far, a single digit was affected, either the thumb or the big toe. This fact underlines the role of trauma as an initiator of the process.
Histologically, subungual epidermoid inclusions appear as epithelial buds and bulbous proliferations originating from tips of the rete ridges of nail bed epithelium and distal nail matrix epithelium [3,4,31]. All cases are characterized by formation of multiple epidermoid microcysts that lose their connection to the overlying nail bed epithelium and come to involve the superficial and sometimes even the deep dermis of the nail bed. The multiple microcysts may be accompanied by solid islands composed of bland keratinocytes. The epithelial proliferation can be limited to a few epithelial rete ridges of the nail bed or can involve a large portion of it or even the whole nail bed [3,4]. The nail bed epithelium usually shows hyperkeratosis and acanthosis. In general, the microcysts show an abrupt keratinization without a granular layer, similar to that encountered in the outer root sheath of the follicular isthmus, the catagen follicle and the isthmus catagen (trichilemmal) cyst [3,30,33]. Sometimes, the epidermoid inclusion cysts show hybrid features between onycholemmal/tricholemmal and infundibular microcyst displaying a thin discontinuous granular layer [4], like in our case (fig.3c and 3d). Some microcysts may become calcified [31]. Recently, Perrin [4] described presence of microductal structures with multivacuolated cells, clear cells, and mucin in the lumen of the ducts in tricholemmal/onycholemmal microcysts. These particular findings, together with the observation that some of the microcysts display infundibular keratinization, made Perrin postulate that this condition represents in fact a benign folliculo-apocrine unit tumor or a hamartoma originating from vestigial follicular units of the nail bed.4 Whether subungual epidermoid inclusions represent a reactive process or a neoplastic condition is still a debated matter [3,4,34].
Subungual melanoma development in association with subungual epidermoid inclusions has never been fully characterized before. Fanti and Tosti [3] and Perrin [4] respectively, briefly mention the existence of this association. However, they do not substantiate this observation with photographic documentation.
We consider that in our case the melanocytic proliferation developed from the beginning in association with the epithelial one. One can argue that the presence of subungual epidermoid inclusions in the nail bed dermis might represent an accidental epithelial implantation from previous surgery. However, pathological findings were present along the entire midline portion of the nail bed corresponding to the longitudinal nail plate splitting.
This peculiar association, that we consider to be site specific, can be a potential pitfall for interpretation and diagnosis, as it may be misinterpreted as a collision tumor between a melanoma and a squamous cell carcinoma or an onycholemmal carcinoma. However, the absence of architectural disarray, nuclear atypia, dyskeratotic cells, large glassy spinous cells and confluent parakeratosis of the discrete roundish epithelial islands dispersed in the dermis of the nail bed excludes this possibility in our case. Also, onycholemmal carcinoma is an asymmetric, poorly circumscribed and deeply invasive tumor composed of trycholemmal microcysts and small solid aggregations of well differentiated basaloid to squamoid cells exhibiting at least focal cytological atypia [4,30]. Lastly, a passing consideration in the histological differential diagnosis would be onychocytic matricoma that has a superficial resemblance to an irritated seborrheic keratosis. However, onychocytic matricoma is a benign tumor of the nail matrix with a different overall architecture being relatively well demarcated at its base forming a straight border and being composed of a basal compartment with a varying admixture of prekeratogenous and keratogenous cells [35]. Clinically though, pigmented onychocytic matricoma can be mistaken for a melanoma.
In conclusion, this present case report is of a subungual melanoma that developed in association with exuberant subungual epidermoid inclusions. This could represent a site specific association. In nail apparatus melanoma biopsies, areas of melanoma in situ in the nail matrix can be seen adjacent to areas of obvious invasive melanoma at the level of the nail bed dermis. Subungual melanoma growth along solid epithelial islands and nail bed microcysts imposes difficulties in the measurement of Breslow thickness with important consequences on classification and management of this tumor.
References
- Mott RT, Rosenberg A, Livingston S, Morgan MB (2002) Melanoma associated with pseudoepitheliomatous hyperplasia: a case series and investigation into the role of epidermal growth factor receptor. J Cutan Pathol 29: 490-497.[Crossref]
- Izumi M, Ohara K, Hoashi T, Nakayama H, Chiu CS et al. (2008) Subungual melanoma: histological examination of 50 cases from early stage to bone invasion. J Dermatol 35: 695-703. [Crossref]
- Fanti PA, Tosti A (1989) Subungual epidermoid inclusions: report of 8 cases. Dermatologica 178: 209-212.[Crossref]
- Perrin C (2013) Tumors of the nail unit. A review. Part II: acquired localized longitudinal pachyonychia and masked nail tumors. Am J Dermatopathol 35: 693-709.[Crossref]
- Dawber RP, Colver GB (1991) The spectrum of malignant melanoma of the nail apparatus. Semin Dermatol 10: 82-87.[Crossref]
- Finley RK 3rd, Driscoll DL, Blumenson LE, Karakousis CP (1994) Subungual melanoma: an eighteen-year review. Surgery 116: 96-100.[Crossref]
- Banfield CC, Redburn JC, Dawber RP (1998) The incidence and prognosis of nail apparatus melanoma. A retrospective study of 105 patients in four English regions. Br J Dermatol 139: 276-279.[Crossref]
- Kiryu H (1998) Malignant melanoma in situ arising in the nail unit of a child. J Dermatol 25: 41-44.[Crossref]
- Phan A, Touzet S, Dalle S, Ronger-Savl S, Balme B, et al. (2006) Acral lentiginous melanoma: a clinicoprognostic study of 126 cases. Br J Dermatol 155: 561-569.[Crossref]
- Saida T, Ohshima Y (1989) Clinical and histopathologic characteristics of early lesions of subungual malignant melanoma. Cancer 63: 556-560.[Crossref]
- Saida T (1992) Heterogeneity of the site of origin of malignant melanoma in ungual areas: 'subungual' malignant melanoma may be a misnomer. Br J Dermatol 126: 529.[Crossref]
- Tan KB, Moncrieff M, Thompson JF, McCarthy SW, Shaw HM, et al. (2007) Subungual melanoma: a study of 124 cases highlighting features of early lesions, potential pitfalls in diagnosis, and guidelines for histologic reporting. Am J Surg Pathol 31: 1902-1912. [Crossref]
- Haneke E (2011) Advanced nail surgery. J Cutan Aesthet Surg 4: 167-175.[Crossref]
- Lee WJ, Lee JH, Won2021 Copyright OAT. All rights reserv Nail apparatus melanoma: A comparative, clinicoprognostic study of the initial clinical and morphological characteristics of 49 patients. J Am Acad Dermatol 73: 213-220. [Crossref]
- Baran R, Kechijian P (1996) Hutchinson's sign: a reappraisal. J Am Acad Dermatol 34: 87-90.[Crossref]
- André J, Moulonguet I, Goettmann-Bonvallot S (2010) In situ amelanotic melanoma of the nail unit mimicking lichen planus: report of 3 cases. Arch Dermatol 146: 418-421.[Crossref]
- Amin B, Nehal KS, Jungbluth AA, Zaidi B, Brady MS, et al. (2008) Histologic distinction between subungual lentigo and melanoma. Am J Surg Pathol 32: 835-843.[Crossref]
- Spencer JM (1999) Nail-apparatus melanoma. Lancet 353: 84-85.[Crossref]
- Milton GW, Shaw HM, McCarthy WH (1985) Subungual malignant melanoma: a disease entity separate from other forms of cutaneous melanoma. Australas J Dermatol 26: 61-64.[Crossref]
- Park SW, Jang KT, Lee JH, Park JH, Kwon GY, et al. (2016) Scattered atypical melanocytes with hyperchromatic nuclei in the nail matrix: diagnostic clue for early subungual melanoma in situ. J Cutan Pathol 43: 41-52.[Crossref]
- Shin HT, Jang KT, Mun GH, Lee DY, Lee JB4 (2014) Histopathological analysis of the progression pattern of subungual melanoma: late tendency of dermal invasion in the nail matrix area. Mod Pathol 27: 1461-1467.[Crossref]
- Viros A, Fridlyand J, Bauer J, Lasithiotakis K, Garbe C, et al. (2008) Improving melanoma classification by integrating genetic and morphologic features. PLoS Med 5: e120.[Crossref]
- Alexeev V, Yoon K (2006) Distinctive role of the cKit receptor tyrosine kinase signaling in mammalian melanocytes. J Invest Dermatol 126: 1102-1110.[Crossref]
- Whiteman DC, Pavan WJ, Bastian BC (2011) The melanomas: a synthesis of epidemiological, clinical, histopathological, genetic, and biological aspects, supporting distinct subtypes, causal pathways, and cells of origin. Pigment Cell Melanoma Res 24: 879-897. [Crossref]
- SAMMAN PD (1959) The human toe nail. Its genesis and blood supply. Br J Dermatol 71: 296-302.[Crossref]
- Lewin K (1965) The finger nail in general disease. A macroscopic and microscopic study of 87 consecutive autopsies. Br J Dermatol 77: 431-438.[Crossref]
- Lewin K (1969) Subungual epidermoid inclusions. Br J Dermatol 81: 671-675.[Crossref]
- Sáez-de-Ocariz MM, DomÃnguez-Cherit J, GarcÃa-Corona C (2001) Subungual epidermoid cysts. Int J Dermatol 40: 524-526.[Crossref]
- Takiyoshi N, Nakano H, Matsuzaki Y, Aizu T, Kaneko T, et al. (2009) An eclipse in the subungual space: a diagnostic sign for a subungual epidermal cyst? Br J Dermatol 161: 962-963.[Crossref]
- Alessi E, Coggi A, Gianotti R, Parafioriti A, Berti E (2004) Onycholemmal carcinoma. Am J Dermatopathol 26: 397-402.[Crossref]
- Telang GH, Jellinek N (2007) Multiple calcified subungual epidermoid inclusions. J Am Acad Dermatol 56: 336-339.[Crossref]
- Bukhari IA, Al-Mugharbel R (2004) Subungual epidermoid inclusions. Saudi Med J 25: 522-523.[Crossref]
- Ackerman AB (1978) Histologic diagnosis of inflammatory skin diseases. Lea & Febiger, Philadelphia. Pp. 83.
- André J, Sass U, Richert B, Theunis A (2013) Nail pathology. Clin Dermatol 31: 526-539. [Crossref]
- Perrin C, Cannata GE, Bossard C, Grill JM, Ambrossetti D, et al. (2012) Onychocytic matricoma presenting as pachymelanonychia longitudinal. A new entity (report of five cases). Am J Dermatopathol 34: 54-59.[Crossref]



