Background: Despite intensive studies of atherosclerosis associated with DM, the mechanisms of the close relationship between diabetes and CAD are not yet fully indicated; as a result, the search and study of other causes, including vascular inflammatory processes, that accelerate and aggravate the development of atherosclerotic vascular lesions and complications is intensively continuing and that identify the relevance of the research problem.
Aim: To perform comparative analysis of lipid profile and markers of vascular inflammatory reaction in patients with CAD and stable angina in general groups and identifying the subgroups with significant coronary artery stenosis without and with DM 2 type. To estimate biochemical parameters of blood serum prospectively after angioplasty in subgroups of patients with significant coronary artery stenosis.
Materials and methods: 97 patients (males and females) aged 60.3 ± 9.8 years with CAD and stable effort angina were studied. Patients were divided into two groups: group 1 (n=64) with CAD, group 2 (n=33) CAD patients with DM 2 type (n=33). According to selective coronary angiography patients in both groups had significant stenosis of one of the coronary arteries with the degree ≥75% of the vessel lumen. Patients with significant coronary artery stenosis in both subgroups underwent coronary angioplasty with the one-type stents with a drug coating. Study of blood plasma laboratory parameters was conducted in the groups of patients initially at the admission before coronary angiography and at the control points – 3, 6 and 12 months after angioplasty and stenting. Duration of patients follow-up was 12 ± 1.2 years after angioplasty.
Results: In both groups of patients, the excess of the reference values of the lipid profile atherogenic parameters (TC, LDL, VLDL, TG) was detected. Significant excess of the level of vascular inflammation markers (hs-СRP, TNF –α, homocysteine, IL-1β) was registered along with the excess of normal values of MMP-9 and endothelin-1 in the group of patients with CAD with DM type 2 that could indicate more pronounced vascular inflammation in this group. Prospective follow-up showed the maximum increased level of inflammatory markers at the control point "3 months" after angioplasty in both subgroups. In general, within the positive dynamics of the studied parameters, no attainments in target lipid levels (TC, HDL, TG) and markers of inflammation (HF-CRP, MMP-9) were found at the endpoint of follow-up "12 months" that could indicate the persistence of a systemic slow vascular inflammatory response.
Conclusions: Systemic inflammatory response is more pronounced in CAD plus DM type 2 patients both in the general group and in a subgroup with significant coronary stenosis. Maximum level of activation of vascular inflammation parameters at the point "3 months" after angioplasty and persistence of a systemic slow vascular inflammatory response to the endpoint of follow-up determine the patients of both subgroups as patients of very high cardiovascular risk and suggest that they have an equivalent potential in the development of both early restenosis and late atherothrombotic complications.
diabetes mellitus type 2, сoronary artery disease, significant coronary stenosis, lipid profile, inflammatory markers
Diabetes mellitus (DM) have been steadily increasing all over the world and nowadays DM is one of the most common non-communicable diseases. The disease has been expanding rapidly: according to up-to-date reports, number of patients with diagnosed DM worldwide increased more than twice over the last 10 years and by end 2014 had risen to a staggering 387 million. According to data of National register of diabetes mellitus in Russian Federation, 4.1 million patients with diabetes were recorded as of January 2015, and the prevalence of patients with type 2 DM (T2DM) was 3.7 mln. [1]. Meanwhile, the data of epidemiologic studies conducted in Russia in the period 2002–2010 years indicated that the real prevalence of the disease was 3–4 times higher compared to the official registered number of patients and in fact was 9–10 mln. that amounts about 7% of the population. The total number of people with diabetes is projected to rise to 438.7 million in 2030 that would perform 7.7% of the adult population [2,3]. At present the problem of DM disease and its complications step forward among the major issues of world healthcare organizations [4].
Type 2 diabetes increases the risk of coronary heart disease (CAD) events at least by two - to fourfold in type 2 diabetic subjects compared with nondiabetic subjects. In DM the risk of cardiovascular complications rises by 4-6 times that leads to 75% of hospitalization and 80% of deaths in patients with DM [2]. A number of major studies including Framingham Heart Study (1979), Multiple risk factor Intervention trial (MRFIT) (1993), Multinational monitoring of trends and determinants of cardiovascular disease (MONICA) and Thrombolysis in Myocardial Infarction (TIMI)(1997-2006 years) reported that DM increased the risk of coronary heart disease by 66% in men and 203% in women; DM is independent of cholesterol, smoking and blood pressure by a factor of coronary risk; 5-years mortality after myocardial infarction (MI) and mortality of patients with unstable angina and MI without ST segment elevation were twice higher in the DM group of patients compared to patients without DM. According to pathomorphological and angiographic data for CAD in patients with DM was observed more typical (three-vessel disease), diffuse lesion (multisegmental lesion in one artery) and distal coronary artery disease, besides, atherosclerosis in type 2 diabetes is often multifocal and along with coronary arteries extends to other arterial basins. [2,5]
Despite intensive studies of atherosclerosis associated with DM, the mechanisms of the close relationship between diabetes and CAD are not yet fully indicated; as a result, the search for other causes that accelerate and aggravate the development of atherosclerotic vascular lesions and complications when combined with clinical conditions has been extensively conducted [2,6]. Nowadays there is no doubt that in the pathogenesis of vascular lesions in atherosclerosis the important role play factors of inflammation and procoagulants circulating in blood. The role of chronic inflammatory process is under active discussion. DM is associated with an increase in the level of markers of subclinical systemic inflammation; moreover, it is known that the concentration of markers of inflammation such as CRP, cytokines, fibrinogen and peripheral blood leukocytes is higher than in CAD patients without DM [6,7]. Over the past decades, insulin resistance (IR) is not only considered as a risk factor for atherosclerosis, but also similarity in the pathogenesis between the two conditions is described. It is suggested that atherosclerosis and IR have similar pathophysiological mechanisms mainly due to the action of the two major pro-inflammatory cytokines: tumor necrosis factor-α and IL-6 [8,9]. Along with generally recognized and widely used laboratory markers, such as CRP, homocysteine, endothelin-1 and cytokines, recently, in literature much attention has been paid to new biochemical factors of inflammatory reaction such as matrix metalloproteinases (MMPs), inhibitors of matrix metalloproteinase (TIMP) activity, "CD40 ligand/CD40 receptor" system of signal molecules and other playing an important part in initiating early atherosclerotic changes, their progression and development of acute thrombotic complications [10]. Increased level of inflammatory reaction markers, endothelial dysfunction is a factor of increased risk of development of acute atherothrombotic events, especially after a percutaneous intervention both in patients with CAD and in those with CAD and DM [1]. The degree of systemic inflammation activity in patients with CAD and DM can be considered as the most important processes characteristic leading to accelerated development of vessel wall damage and destructive changes in atherosclerotic plaques that identify the relevance of the problem under discussion.
97 patients (males and females) aged 60.3 ± 9.8 years with CAD, stable effort angina and significant coronary artery stenosis were studied. Patients were randomized into two groups: group 1 (n=64) without DM 2 type, group 2 (n=33) with DM 2 type. According to selective coronary angiography patients in both groups had significant stenosis of one of the coronary arteries with the stage ≥75% of the vessel lumen. Study of blood plasma laboratory parameters was conducted in the groups of patients initially at the admission stage before coronary angiography and at the control points – 3, 6 and 12 months after angioplasty and stenting. Duration of patients monitoring after angioplasty was 12 ± 1.2 years.
Study protocol is approved by Ethic committee of the institute. Prior to be included into the study each of the participants provided written informative approval on using study results for the research needs. The study was performed in conditions of real clinical practice during standard therapy of CAD patients which included ACE inhibitors, β- adrenergic blocking agents, calcium antagonists, disaggregants (aspirin, clopidogrel), statins, nitrates for both groups and oral antidiabetic drugs for the patients from group 2. At the pre-admission stage no significant therapy differences between the groups were revealed.
Exclusion criteria were the following: acute coronary syndrome aged less than 30 days, acute cerebral blood flow disorder aged less than 6 months, DM 1 type, congestive heart failure (CAD) of functional class (FC) IV (as per NYHA), oncological and psychiatric diseases.
Diagnostics of CAD forms and comorbidities was performed according to the acting ESC, Society of cardiology of Russian Federation and EASD recommendations. Diagnostic methods included clinical examination, laboratory and instrumental estimation of coronary blood flow (selective coronary angiography).
Coronary angiography was conducted by femoral access using method of M. Judkins (1967) with the help of angiography complexes «Diagnost ARC A», «Poly diagnost C», «Integris Allura»-Phillips-Holland. Amount of injured coronary arteries (CA) and maximum percentage of structure formation was estimated.
Venous blood sampling is performed fasted into disposable tubes of Vacuette system (Japan); the blood was centrifugated during 15 minutes at 2500 rpm by Sigma (Germany). Blood plasma was aliquoted for further freezing (at minus 70 degrees).
Parameters of lipids profile were studied with Cobas Integra 400 plus (Switzerland). Determination of total cholesterol (TC), triglycerides (TG), HDL, LDL, VLDL was performed by direct enzymatic colorimetric method; Concentrations of apolipoproteins A-I (Apo A-I), apolipoproteins B (Apo-B), lipoproteins a (Lp (a)) were performed by the method of immune turbidimetry using analytical sets and control materials "Roche Diagnostics Gmb" (Germany).
As biochemical inflammatory markers there were defined: high-sensitive C-reactive protein (hs-CRP, reference values 0-3.0 mg/l) was measured by immune turbidimetry method analytic set «C-reactive protein hs» (BioSystem, Spain) on the semi-automatic open type analyzer Clima MC-15 (Spain); interleukin-1β (IL-1β, reference values 0-5.0 pg/ml), interleukin -6 (IL-6), interleukin -8 (IL-8), tumor necrosis factor – α (TNF-α, reference values 0-8.11 pg/ml) – «sandwich» and homocysteine (HYC, reference values 5.0-15.0 mcmol/l) were measured by competitive methods (solidphase chemiluminescent immunoenzymometric analysis) on the analyzer IMMULITE 1000 (Siemens Diagnostics, USA); dissolvable CD40 - ligand (sCD40 L) was measured by «immunoenzymometric sandwich» using sets Human sCD40L Elisa on the analyzer Bender MedSystems, Austria; receptor CD40 and matrix metalloproteinase -9 (MMP-9, reference values 20.3-77.2 ng/ml) was measured by Bender MedSystems an eBioscience company, Austria; tissue inhibitor of metalloproteinase -1 (TIMP-1, reference values 92-116 ng/ml)) – was measured by Human TIMP-1 Elisa K.t Invitrogen, USA on the analyzer Personal Lab, Italy.
Carbohydrate metabolism was estimated at the content of glucose and glicated hemoglobin (НbAlc). Glucose concentration in blood was defined by hexokinase method on the biological analyzer «Cobas Integra 400 plus». Glicated hemoglobin was defined by chromatographic method using analyzer Bio-Rad D10, USA.
Atherogenic index (AI) = Апо В/Апо А-I was revealed by calculation.
Values of endothelium functional activity in blood plasma: nitrites level (reference value 3.77 ± 0.87 nmol/l) was defined on the biochemical analyzer Humalyzer 2000 Human (Germany, 1995) and endothelin-1-21 level (reference values 0.2-0.7 fmol/l) was defined on the semi-automatic immunoenzymometric analyzer «Dynatech» (Germany, 1989 г).
Blood sampling was performed out of peripheral vein initially at the admission stage (before coronary angiography).
Statistical data processing was conducted using application software package Stаtistiса (SPSS Inc, vеr 11.5). Kolmogorov-Smirnov’s test was applied for the test of hypothesis about distribution normality. Student's t-test was used between the groups to discover differences in the numerous variables of normal distribution; non-parametric Mann–Whitney test was used for comparison of qualitative and quantitative values not being normal. Comparison of the groups was done using Wilcoxon test for paired measurements. The data was presented as М ± SD - «average value ± standard deviation», р<0.05. Estimation of association between characteristics subject to normal and abnormal distributions was carried out using the Pearson and Spearman rank correlation coefficients, respectively.
Patients of group 1 and 2 included in the study did not have significant differences (р>0.05) in age (60.15 ± 8.57 and 60.39 ± 9.51 years), gender (males 32.8% and 54.5%, females 45.5% and 67.2%), CAD history (7.69 ± 6.38 and 9.97 ± 7.67 years), earlier myocardial infarction in past medical history (males 59.4% and 63.6%, females 40.6% and 36.4%), having comorbidities such as arterial hypertension, dyslipidemia, smoking. The majority of patients in both groups had effort angina II FC (64.1% and 51.5%) and obesity I class (53.1% and 42.4%), respectively. The groups were significantly distinguished by multivessel diseases with the proved excess in the group with DM 2 type (6.3% and 21.2%, respectively, р=0.029).
Biochemical values in general groups of patients with CAD with and without DM 2 type at the initial study stage are given in the Table 1.
Table 1. Biochemical values in general groups of patients with CAD with and without DM 2 type at the initial study stage (М ± SD).
Values |
CAD patients without DM 2 type (n=64) |
CAD patients with DM (n=33) |
р |
Lipid profile: |
TC (mmol/l) |
4.97 ± 1.26 |
5.52 ± 1.00 |
0.032 |
HDL (mmol/l) |
1.14 ± 0.27 |
1.05 ± 0.19 |
0.080 |
LDL (mmol/l) |
2.89 ± 1.05 |
3.09 ± 0.76 |
0.338 |
TG (mmol/l) |
2.12 ± 0.99 |
2.38 ± 1.26 |
0.376 |
VLDL (mmol/l) |
0.76 ± 0.33 |
0.85 ± 0.33 |
0.203 |
Lp-(a) (mg/dl) |
30.96 ± 29.17 |
20.57 ± 19.94 |
0.042 |
Apo -A1(mg/dl) |
151.61 ± 26.18 |
154.43 ± 24.80 |
0.611 |
Ap-B (mg/dl) |
86.61 ± 21.97 |
93.49 ± 23.83 |
0.159 |
Inflammatory markers: |
hs-CRP (mg/l) |
2.73 ± 1.19 |
3.69 ± 1.14 |
0.001 |
TNF-α (pg/ml) |
9.52 ± 3.21 |
11.00 ± 4.10 |
0.043 |
Homocysteine (mcmol/l) |
11.45 ± 5.40 |
15.93 ± 7.14 |
0.003 |
IL-1v (pg/ml) |
4.27 ± 1.37 |
5.22 ± 1.83 |
0.003 |
IL -6 (pg/ml) |
3.12 ± 2.02 |
4.65 ± 3.37 |
0.097 |
IL -8 (pg/ml) |
16.55 ± 12.72 |
18.91 ± 15.45 |
0.703 |
СD 40 (ng/ml) |
94.01 ± 40.43 |
96.85 ± 44.38 |
0.761 |
sCD40L (ng/ml) |
3.49 ± 0.99 |
3.31 ± 1.08 |
0.430 |
TIMP -1 (ng/ml) |
86.93 ± 17.44 |
85.31 ± 11.52 |
0.631 |
ММР-9 (ng/ml) |
89.74 ± 31.36 |
87.11 ± 30.56 |
0.964 |
Endothelial dysfunction: |
Endotheline -1 (fmol/l) |
1.06 ± 0.43 |
0.93 ± 0.49 |
0.188 |
Nitrites (nmol/l) |
2.89 ± 1.30 |
2.84 ± 1.29 |
0.843 |
Carbohydrate metabolism: |
Blood glucose (mmol/l) |
5.38 ± 0.75 |
7.65 ± 2.03 |
0.001 |
Glicated hemoglobin (%) |
5.5 ± 0.50 |
7.1 ± 1.00 |
0.000 |
Note: n- amount of patients, p – significance of differences.
Estimation of lipid profile showed significant excess of TC (р=0.032) in the group with DM 2 type. Besides, excess of reference values of such atherogenic parameters as LDL, TG, VLDL in both groups of patients and significant excess of Lp (a) (р=0.042) in group 1 were noticed.
It is well known that DM 2 type having special quantitative changes also has important qualitative changes in lipid profile, which additionally enhanced its atherogenic potential. In the result of nonenzymatic glycosylation of apoproteins disorder of LDL clearance and collecting of small solid elements of this class is happening. These changes of lipoproteins structure are considered as one of the most important reasons of accelerated development of atherosclerosis with DM 2 type. [11]
Hypertriglyceridemia (HTG) is a more rigid marker of CAD in patients with DM 2 type than in people without hyperglycemia. As per the data of 11 years prospective DM patients monitoring (Paris Prospective Study), level of blood TG was connected with death risk of CAD. According to opinion of M. Laakso and co-authors important prognostic meaning of disease and death of CAD and its complications in patients with DM 2 type lies in lowering of HDL, decrease of which less than 0.9 mmol/l is accompanied by 4 times increase of death risk of cardiac pathology. [11] According to our study trend of lowering of HDL with trend to increase of TG, LDL and VLDL is revealed in group of patients with DM 2 type.
Having direct correlational interrelations of average power (r=0.4-0.5) between glicated hemoglobin and values of atherogenic dyslipidemia: TC (p=0.02), VLDL (p=0.04), Apo-B (p=0.01) and TG (p=0.003) among patients with DM 2 type proves that patients of group 2 have diabetic dyslipidemia. The last one is a specific variant of atherogenic dyslipidemia promoted atherosclerosis not depending on TC increase and total fraction of LDL. Diabetic dyslipidemia relates to the risk of CAD as well as isolated mild hypercholesterolemia [12].
Mean value of LP (a) in group 1 and 2 was on the border level (30.96 ± 29.17 and 20.57 ± 19.94 mg/dl, respectively) but the fact of the possibility of cardiovascular accidents and due to structural similarity of Lp (a) with plasminogen allows to regard it as competitive antagonist of the last one and can be associated with excess risk of coronary thrombosis. According to retrospective studies exceeded levels of Lp (a) are connected with progress of atherosclerotic plaques in the coronary arteries, which never had stenosis before [13,14].
It is considered that chronic subclinical inflammation is a part of insulin resistance syndrome and cytokines are predictors of vascular complications of DM 2 type [9,15,16].
The second group of patients had considerable hyperactivation of markers of system and local inflammation responds: hs-СRP (р=0.001), homocysteine (р=0.003), TNF-α (р=0.043). Levels of IL-6 and IL-8 had a tendency to values rise, within standard values, in comparison with group 1.
Besides, group with DM 2 type has significant excess of cytokine IL-1β (p=0.003) which is the main mediator responsible for local inflammatory response and acute phase response of the organism. According to the literature coronary blood flow disorder and myocardial ischemia leads to increase of its content in blood. Also, there is information about no system activation of Il-1β in case of stable and unstable angina. There is opinion that initiation of smoldering inflammation connected with rise of basic hs-СRP leads to insulin resistance and inductors of inflammation are proinflammatory cytokins, specially interleukins IL-6 and IL-1β [16-18].
Today increase of homocysteine in blood plasma is considered as significant risk factor of atherosclerotic vascular disease. It is supposed that homocysteine is subjected to autoxidation forming free radicals which damage vascular endothelium with further endothelium dysfunction. It starts a complicated set of enzymatic reactions leading to induction of TC synthesis and LDL oxidation, which stimulates atherogenic processes [19,20]. More than 80 clinical and epidemiological studies have been performed and it was confirmed that HTG is one of the most important and independent risk factors of early and quick progress of atherosclerosis and coronary thrombosis. Clinical studies revealed that due to the oxidation stress HTG encourages insulin resistance and beta cells dysfunction accelerating DM progress [21]. Besides, Fonseca V. et al. in his rat study showed that increase of homocysteine concentration in blood may appear with hyperinsulinemia in the result of insulin injection; it may cause the vicious circle in case of insulin dependent DM.
Using method of binary logistic regression it was revealed that patients of group 1 had risk of DM 2 type increased for 1.1 times (odd ratio=1.115; 95%-confidence interval 1.011-1.229; р=0.03) due to growth of homocysteine for 1 mcmol/l; in conditions of elevated hs-СRP more than 3 mg/l and its increase for 1 mg/l a chance of having DM 2 type raises for 3.8 times (OR=3.819;95%- confidence interval 1.551-9.404; р=0.004).
In groups 1 and 2 elevated but significantly not different values of local inflammatory reactions – MMP-9 and lowering of TIMP-1 were revealed; it corresponds results of experimental and clinical studies showing tendency to growth of proinflammatory cytokines and proteinas with decrease of activity of anti-inflammatory mediators in case of DM 2 type [22].
Among signal molecules participating in immune reactions and inflammation, an important role belongs to the system «receptor CD 40 - ligand CD40». Their expression is found in lymphocytes, monocytes, thrombocytes, endothelial cells, β cells of insula of pancreatic gland, adipocytes and other tissues. System «receptor CD 40-L CD40» participates in forming if immune inflammatory responses in cardiovascular system and thrombosis. Signals realized through receptor CD 40 are involved in atherosclerosis and diabetic nephropathy, this allowed to regard this system as a universal element of pathogenesis uniting inflammatory disorders, hyperglycemia and vascular complications DM [10].
Among indicators of signal inflammatory system (CD 40, sCD40L) our study did not find any significant differences and elevated values in both groups; it could possibly be connected with lack of patients.
Besides, it should be noted that prognostic importance of the values used today for estimation of endothelial function is not entirely studied. It is supposed that in case of DM 2 type hyperhomocysteinemia can confound endothelial dysfunction, accelerate atherosclerotic processes, raise oxidative stress, lower thromboresistance, raise aggregative platelets ability and their adhesive features. Experimental and clinical studies showed that patients with DM 2 type had endothelial dysfunction. Hyperglycemia activates protein kinase C in endothelial cells, it may cause production of vasoconstrictive prostaglandins, endothelin-1 and angiotensine transforming enzyme which have direct or indirect harmful impact on vasomotor response [20].
Our study did not reveal any significant difference in endothelium 1-21 and nitrites in the main groups of patients, however, value of endothelium-1 was higher and nitrites value was lower than reference values in both groups of patients.
Using correlation analysis in the groups under study numerous interrelations were revealed (r>0.5). Patients of group 2 had hs-CRP with TC (р=0.004) and Apo-B (р=0.02); IL-1β with TC and TG (р=0.01); endothelin -1 with hs-CRP (р=0.02), TNF-α (р=0.01), MMP-9 (р=0.05); CD 40 with TG (р=0.03), which corresponds the theory about atherosclerosis as a process combining inflammation and thrombosis. Correlations between the history of DM 2 type, body mass index (BMI) (p=0.002) and homocysteine (p=0.02) were noticed. Hyperhomocysteinemia of more than 15 mcmol/l in group 2 was found more often than in the group without DM 2 type and was significantly interrelated with glycemia (р=0.04, r=0.40). Group 1 had direct interrelations of smoking history with TG (p=0.03) and LDL (p=0.03); hs-CRP with homocysteine (p=0.03) and IL 6 (p=0.03).
Next stage of our study had the target to compare the values under study in sub-groups with significant coronary artery stenosis in CAD patients with and without DM 2 type and follow dynamics of the biochemical parameters in the control points after 3, 6 and 12 months after angioplasty and stenting.
Amount of patients after angioplasty and stenting in the CAD group without DM 2 type was 32 (50% of total amount of patients), and in the CAD group with DM 2 type – 22 (66.7%, respectively).
Comparative characteristics of the main biological values between the groups of patients with significant coronary artery stenosis in CAD patients with and without DM 2 type initially and characteristics of the values in dynamics in a year after angioplasty are presented in Table 2.
Table 2. Characteristics of biochemical values in sub-groups of CAD patients with and without Dm 2 type having significant coronary artery stenosis at the final stage of the study and in a year after angioplasty (М ± SD).
Values |
CAD patients with significant coronary artery stenosis (n=32) |
CAD and DM patients with significant coronary artery stenosis (n=22) |
р |
Lipid profile: |
TC (mmol/l) |
initially |
5.26 ± 1.31 |
5.44 ± 1.00 |
0.549 |
a year after |
4.62 ± 0.99 |
4.92 ± 1.07 |
0.393 |
р |
0.016 |
0.014 |
|
HDL (mmol/l) |
initially |
1.09 ± 0.26 |
0.97 ± 0.16 |
0.057 |
a year after |
1.21 ± 0.28 |
1.16 ± 0.17 |
0.797 |
р |
>0.05 |
0.001 |
|
LDL (mmol/l) |
initially |
3.09 ± 1.05 |
3.18 ± 0.75 |
0.526 |
a year after |
2.42 ± 0.87 |
2.56 ± 0.95 |
0.560 |
р |
0.001 |
0.001 |
|
TG (mmol/l) |
initially |
2.29 ± 1.03 |
2.23 ± 1.12 |
0.586 |
a year after |
1.70 ± 0.36 |
1.91 ± 0.64 |
0.640 |
р |
0.006 |
>0.05 |
|
VLDL (mmol/l) |
initially |
0.87 ± 0.36 |
0.84 ± 0.32 |
0.819 |
a year after |
0.59 ± 0.246 |
0.73 ± 0.30 |
0.066 |
р |
0.001 |
>0.05 |
|
Lp-(a) (mg/dl) |
initially |
37.38 ± 34.30 |
19.86 ± 22.20 |
0.027 |
a year after |
27.10 ± 27.33 |
24.82 ± 31.06 |
0.515 |
р |
>0.05 |
>0.05 |
|
Apo-B (mg/dl) |
initially |
91.88 ± 21.60 |
92.25 ± 2.32 |
0.853 |
a year after |
90.00 ± 21.82 |
109.12 ± 28.58 |
0.007 |
р |
>0.05 |
0.014 |
|
Inflammatory markers: |
hs-СRP (mg/l) |
initially |
2.87 ± 1.25 |
3.58 ± 1.23 |
0.044 |
a year after |
2.80 ± 0.97 |
2.86 ± 0.82 |
0.660 |
р |
>0.05 |
0.008 |
|
TNF -α (pg/ml) |
initially |
9.44 ± 2.50 |
11.24 ± 4.02 |
0.048 |
a year after |
5.86 ± 3.18 |
7.39 ± 2.84 |
0.042 |
р |
0.001 |
0.01 |
|
Homocysteine (mcmol/l) |
initially |
11.08 ± 5.42 |
17.04 ± 7.52 |
0.09 |
a year after |
9.77 ± 5.88 |
12.20 ± 4.10 |
0.016 |
р |
>0.05 |
>0.05 |
|
IL-1в (pg/ml) |
initially |
4.24 ± 1.06 |
5.57 ± 2.01 |
0.044 |
через год |
4.00 ± 1.30 |
4.49 ± 1.94 |
0.459 |
р |
>0.05 |
0.002 |
|
MMP-9 (ng/ml) |
initially |
86.45 ± 31.01 |
83.50 ± 26.45 |
0.860 |
a year after |
87.77 ± 29.86 |
82.68 ± 33.83 |
0.622 |
р |
>0.05 |
>0.05 |
|
TIMP -1 (ng/ml) |
initially |
91.00 ± 17.22 |
86.38 ± 12.26 |
0.365 |
a year after |
105.70 ± 29.15 |
112.65 ± 26.32 |
0.342 |
р |
>0.05 |
0.002 |
|
Markers of endothelial dysfunction: |
Endotheline -1 (fmol/l) |
initially |
1.07 ± 0.48 |
0.96 ± 051 |
0.423 |
a year after |
0.97 ± 0.47 |
0.93 ± 0.39 |
0.895 |
р |
>0.05 |
>0.05 |
|
Nitrites (nmol/l) |
initially |
3.34 ± 1.23 |
2.63 ± 1.38 |
0.055 |
a year after |
3.45 ± 0.97 |
3.20 ± 0.91 |
0.132 |
р |
>0.05 |
>0.05 |
|
2021 Copyright OAT. All rights reserv
Note: n- amount of patients, p – significance of differences.
Parameters of lipid profile and inflammatory markers in subgroups with significant coronary artery stenosis are characterized by unidirectional changes with general groups of CAD patients with and without DM type 2.
Comparative characteristics of the biological values presented in table 2 showed that in patients of subgroup 2 with significant coronary artery stenosis if there is DM type 2 significantly lower HDL, Lp (a), and nitrite levels and significantly higher levels of hs-СRP, TNF -α, homocysteine and IL-1b were detected for parameters of lipid profile and inflammatory reaction markers that determine higher degree of vascular inflammation intensity in this subgroup. However, the excess of reference values for all studied parameters detected in the first group of patients without diabetes allows to identify patients of both subgroups as patients at very high risk of cardiovascular complications.
A comparative analysis of the levels at the starting point and at the end point of the study showed that among the lipid profile parameters in both subgroups the levels of TC and LDL significantly decreased while in the 1 subgroup additional reductions in TG and VLDL levels was observed and in the 2nd subgroup the increase of HDL and Apo-B levels was detected. In both groups all atherogenic lipid fractions remained at a level higher than the target levels.
In assessing the markers of inflammation, it was revealed that in both subgroups the level of TNF-α significantly decreased. In the 2nd subgroup, the levels of hs-СRP and IL-1в also significantly lowered with an increase of TIMP-1 level. Concentration of markers of endothelial activity and MMP-9 level did not significantly change in both groups remaining at the level above the reference values.
In general, it should be noted that in both subgroups of patients the signs of slow vascular inflammation remain until the end point of follow-up indicating that the groups have an equivalent potential in late atherothrombotic complications. Parameters of carbohydrate metabolism at the end point of follow-up did not significantly change in the CAD group with DM.
Maximum increased level of inflammatory markers (hs-СRP, TNF –α, Homocysteine, IL-1v, IL -6, IL -8, СD 40, sCD40L, ММР-9, TIMP -1) was detected at the point "3 months" after angioplasty in both subgroups of patients with significant coronary artery stenosis. The main data are shown in Figure 1.
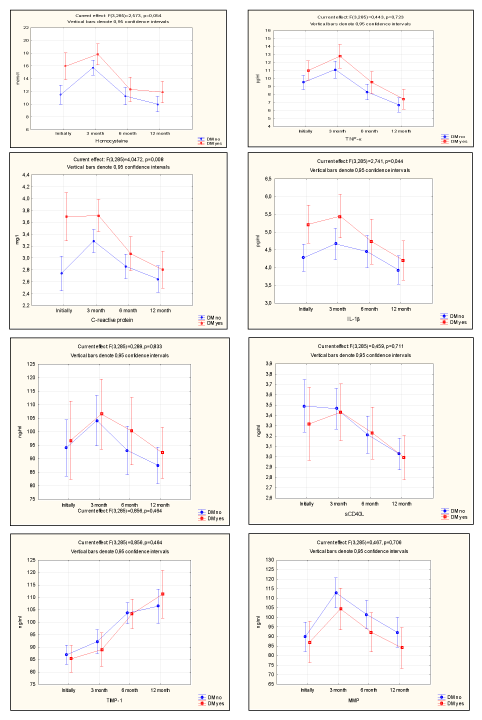
Figure 1. Inflammatory markers growth levels.
Maximum level of activation of vascular inflammation parameters at the point "3 months" after angioplasty determines the patients of both subgroups as patients of high risk for early restenosis and late atherothrombotic complications as a result of a combined cause supporting the process of vascular inflammation.
In recent years, as the frequency of percutaneous coronary interventions with stenting has increased, much attention is focused on solving problems associated with thrombosis and restenosis within the stents. In-stent restenosis (ISR) as a healing process of a damaged vessel after stenting occurs in about 10-40% of patients. The inside stent thrombosis is observed in 0.87-2.2% of cases and develops, as a rule, within the first year after PCI. The main pathogenetic mechanisms of ISR development are the elastic luminal occlusion, parietal thrombus formation and neointimal hyperplasia leading to abnormal vessel wall remodeling. As a starting point, mechanical damage of artery intima-media is considered, as well as hypersensitivity to stent materials. It is well known that vessel wall trauma during the intervention leads to the development of a local inflammatory reaction, adhesion, platelet activation and aggregation with parietal thrombus formation, smooth muscle cell migration and proliferation, rendothelization along with synthesis of extracellular matrix components. All the processes are physiological and necessary for restoration of both anatomical and functional integrity of the vascular wall. However, in some cases they become pathological and lead to neointimal hyperplasia and chronic vasoconstriction. According to some authors` opinion, stenting prevent elastic recoil, but does not prevent the thrombosis, inflammation and neointimal hyperplasia [23].
Characteristic features for patients with CAD combined with DM type 2 are dyslipidemia with elevated total cholesterol (TC) level and low level of high-density lipoprotein cholesterol (HDL), along with hyperactivation of markers of vascular inflammatory response with a significantly higher level of hs-СRP, TNF-a, homocysteine and IL-1β compared to CAD patients without type 2 DM. In subgroups with significant coronary stenosis, significantly increased levels of markers of vascular inflammatory reaction in CAD and type 2 DM as well as excess of these values of reference range for CAD without DM identifies the patients of both subgroups as patients with a very high cardiovascular risk, indicating that they have an equivalent potential in development of cardiovascular complications. A prospective follow-up of patients after angioplasty showed the maximum level of activation of vascular inflammation parameters at the point "3 months" that indicates the probability of high risk of early restenosis. In general, within the positive dynamics of the studied parameters, at the end point of the observation, it was detected that target levels of separate atherogenic lipid fractions (TC, HDL, TG) and markers of inflammation (hs-СRP, MMP-9) were not achieved that may indicate the persistence of a slow vascular inflammatory response with the possibility of late atherothrombotic complications in patients of both groups. The obtained results of the research show the necessity of extensive follow-up programme in ‘real-life’ clinical practice for patients after angioplasty for optimal laboratory control that will reduce the number of vascular complications after stenting, reduce treatment costs and improve the quality of life for patients.
Within the research program of Tyumen Cardiology Research Center, Tomsk National Research Medical Center, Russian Academy of Sciences, Tomsk, Russia.
Authors have no obvious and potential conflicts of interest to declare concerning the publishing of this article.
Tatiana I. Petelina: concept, work design, analysis of results, writing the article; Natalia A. Musikhina: analysis of results, writing the article; Liudmila I. Gapon: analysis of results, writing the article; Vadim A. Kuznetsov: concept of the article; Elena A. Gorbatenko: statistical processing and analysis of the collected data; Irina V. Emenеva: data collection.
- Golukhova EZ, Kuznetsova EV (2016) Myocardial revascularization in patients with coronary heart disease combined with type 2 diabetes mellitus: a review of modern technologies. Diabetes Mellitus 19: 406-413.
- Volkov VI, Serik SA (2011) Diabetes mellitus and coronary atherosclerosis. Atherosclerosis 7: 16-22.
- Shaw JE, Sicree RA, Zimmet PZ (2010) Global estimates of the prevalence of diabetes for 2010 and 2030. Diabetes Res Clin Pract 87: 4-14. [Crossref]
- Dedov II, Shestakova MV, Galstyan GR (2015) Standards of specialized diabetes care. Edited by Dedov II, Shestakova MV (7th edition). Diabetes mellitus 18: 1-112.
- SuminAN, Bezdenezhnykh NA, Bezdenezhnykh AV, et al. (2012) Effect of diabetes of the second type on the prevalence of multifocal atherosclerosis in patients with coronary heart disease. Cardiol 11: 33-41.
- Muir RL (2009) Peripheral arterial disease: Pathophysiology, risk factors, diagnosis, treatment, and prevention. J Vasc Nurs 27: 26-30. [Crossref]
- Bezborodova TA, Tarasov AA, Reznikov, EA, et al. (2014) Diagnostic use of new pathogenic markers of vascular lesions in diabetic patients. Cytokines Inflammation 13: 28-33.
- Titov VN (1999) The generality of atherosclerosis and inflammation: the specificity of atherosclerosis as an inflammatory process. Russian Medical Journal 5: 44-49.
- Gruzdev OV, Barbarash OL, OE Akbasheva (2012) markers of insulin resistance and inflammation in acute myocardial infarction. Cytokines and Inflammation 2: 44-50.
- Shevchenko OP, Prirodova OF, Orlova OV (2006) CD 40 ligand in patients with coronary heart disease combined with type 2 diabetes. Cardiovascular Therapy Prevention 5: 101-111.
- Janashia PH, Mirina EY (2008) Violation of lipid metabolism in type 2 diabetes and its correction options. Russian Medical Journal 11: 1561.
- American Diabetes Association (2003) Position Statement: Management of Dyslipidemia in Adults with Diabetes. Diabetes Care 26: S83-S86.
- Discepolo W, Wun T, Berglund L (2006) Lipoprotein(a) and thrombocytes: potential mechanisms underlying cardiovascular risk. Pathophysiology of Haemostasis and Thrombosis 35: 314-321.
- Hartmann M, von Birgelen C, Mintz GS, Stoel MG, Eggebrecht H, et al. (2006) Relation between lipoprotein(a) and fibrinogen and serial intravascular ultrasound plaque progression in left main coronary arteries. J Am Coll Cardiol 48: 446-452.
- Dehghan A, Kardys I, de Maat MP, Uitterlinden AG, Sijbrands EJ, et al. (2007) Genetic variation, C-reactive protein levels, and incidence of diabetes. Diabetes 56: 872-878.
- Gusev DE, Potievsky BG, Raychevin NA (2012) Markers of inflammation in different forms of coronary heart disease. Cardiology 4: 4-8.
- Klimontov VV, Tian NV, Fazullin O (2016) Clinical and metabolic factors associated with chronic inflammation of low intensity in patients with diabetes mellitus type 2. Diabetes Mellitus 19: 295-302.
- Babayev AR, Tarasov AA, Bezborodov TA (2010) The concept of systemic inflammation in the pathogenesis of diabetic angiopathy. Bulletin of Volgograd State Medical University 1: 308.
- Kravchuk NA (2012) Hyperhomocysteinemia, cardiovascular disease and type 2 diabetes. Health Ukrainy 4: 52-56.
- Davidchek EV, Snezitckyi VA, Niconova LV (2015) Relationship of hyperhomocysteinemia with coronary heart disease and Diabetes mellitus. Education Establishment Grodno State Medical University 1: 9-13.
- Potter K (2008) The oxidant stress of hyperhomocysteinemia. Clin Biochem 45: 27-30.
- Garvin P, Nilsson L, Carstcnsen J (2008) Circulating matrix metalloproteinase-9 is associated with a cardiovascular risk factors in middle-aged normal population. Oxford J Med 101: 785-791.
- Berezovskaya GA, Ganyukov VI, Karpenko MA (2012) Restenosis and thrombosis within the stent: pathogenetic mechanisms of development. Russian Cardiology Journal 6: 91-96.

