Background: Sorafenib is the first systemic therapy approved for hepatocellular carcinoma (HCC), but its efficacy and safety for unresectable HCC remain controversial.
Methods: We searched for randomized controlled trials (RCTs) about sorafenib treatment in unresectable HCC in Web of Science, Embase, PubMed, and the Cochrane Library. Critical outcomes included overall survival (OS) and time to progression (TTP). Important outcomes included disease control rate (DCR), stable disease (SD), and partial response (PR). Hazard ratio (HR) was used to express the effect size of survival data, while dichotomous data were expressed by odds ratio (OR). Safety was measured by relative risk (RR).
Results: Six RCTs with a total of 1751 patients were included in the analysis. We found that sorafenib significantly improved the OS (HR: 0.73; 95%CI: 0.63, 0.85; P<0.001) and DCR (OR: 1.67; 95%CI: 1.14, 2.46; P<0.05) of patients with unresectable HCC, but did not significantly improve TTP. The evidence recommendation of OS and DCR results were high according to the GRADE profile. Sorafenib therapy could increase the incidence of severe drug-related adverse events, but the risk was tolerable.
Conclusion: Sorafenib treatment benefited patients with unresectable HCC in term of OS and DCR, and was comparatively safe in the management of unresectable HCC.
sorafenib, transarterial chemoembolization, unresectable hepatocellular carcinoma, efficacy, safety
Abbreviations:
OS: overall survival; TTSP: time to symptomatic; TTRP: time to radiologic progression; DCR: disease control rate; TTP: time to progression; PFS: progression free survival.
Hepatocellular carcinoma (HCC) is the third leading cause of cancer-related death among men worldwide [1]. In China, approximately 90% of HCC patients have an underlying hepatitis B virus (HBV) infection, and most patients are diagnosed with intermediate or advanced HCC according to histological diagnosis [2-4]. The Barcelona Clinic Liver Cancer (BCLC) stage system recommends treating patients with intermediate HCC with transarterial chemoembolization (TACE), and advanced HCC with sorafenib [5]. That is, these patients cannot benefit from curative treatments such as radical resection, radiofrequency ablation, and liver transplantation. Patients with advanced HCC have a 5-year survival rate of about 15-40% [6]. In 2008, Sorafenib became the first systemic therapy approved by the Food and Drug Administration (FDA) of America for advanced HCC. Sorafenib blocks the activities of the receptor tyrosine kinases of the vascular endothelial growth factor receptors and the serine-threonine kinase Raf-1, thus inhibiting of the carcinogenesis and development of HCC [7].
The FDA’s approval was based on two randomized controlled trials demonstrating that patients with advanced HCC treated with sorafenib showed better survival outcomes than those treated with placebo [8,9]. In the past several years, a large number of clinical trials have investigated the efficacy and safety of sorafenib [8-16], and several systematic reviews concluded that patients with advanced HCC could benefit from sorafenib treatment [17-20]. However, the reviews differed with regard to the pooled overall survival and time to progression. Peng et al reported that sorafenib significantly improved the overall survival and time to progression(TTP) of advanced HCC [18], while Wang et al demonstrated that TTP was significant longer in the sorafenib plus TACE group than in the TACE alone group, but differences in overall survival between the two groups did not reach significance [21]. This systematic review aims to investigate the efficacy and safety of sorafenib in the management of unresectable HCC, and uses the GRADE system to evaluate the evidence value.
Search strategy
We performed an electronic search of Web of Science, Embase, PubMed, and the Cochrane Library. (Sorafenib OR Nexavar) and (hepatic OR hepatocellular OR liver) were used as key words without time limitation. Only manuscripts published in English were included. Both abstracts, full-text and reference lists were thoroughly examined to guarantee the completeness of literature searching. The deadline of searching process was July 2016.
Inclusion criteria
Study design: phase II or III randomized controlled trials(RCTs). Patients: diagnosed with unresectable HCC (advanced HCC, HCC with metastasis or extrahepatic invasion) with perform status (PS 0-2), Child-Paugh A or B, and tolerable renal function and hematologic function who had not received any systematic therapy. Intervention: experimental group: sorafenib or sorafenib combined therapy; control group: placebo or placebo plus the same therapy as the experimental group, without sorafenib.
Exclusion criteria
Studies comparing sorafenib with other systemic drugs for HCC, studies without adequate data, phase I clinical trials, patients who received regional or systemic therapy before the trial, and patients with severe renal and hematologic dysfunction.
Quality assessment of included studies
Two investigators independently evaluated the quality of included studies according to the instructions of Cochrane Handbook for Systematic Review of Interventions [22]. Each study was assessed using the following aspects: random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias (Figure 2).
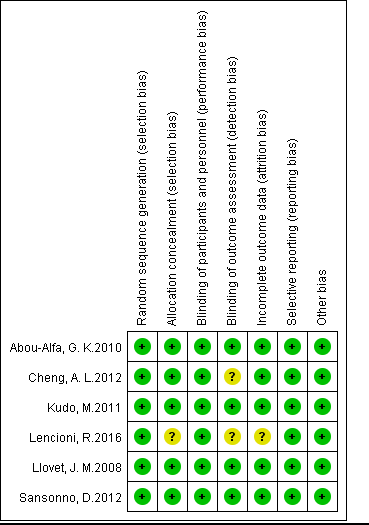
Figure 2. Risk of bias summary
Data extraction: Two investigators abstracted the data, collecting the following data: patients’ region, number, group assigned, age, ratio of patients with Child A liver function, PS grade, Barcelona Clinic Liver Cancer(BCLC )stage, intervention, effect and safety index. Any disagreements were settled through consultation with a third investigator.
Statistics
We divided the endpoints into key outcomes and important outcomes. The key outcomes included overall survival and time to progression. The important outcomes included disease control rate, partial response rate, and stable disease. Tumor response was assessed using the Response Evaluation Criteria in Solid Tumors (RECIST) guidelines [23]. The effect size of survival data was expressed by hazard ratio (HR), and the effect size of dichotomous data was represented by odds ratio (OR). The effect size of safety was expressed by relative risk (RR). All effect sizes were expressed as 95% confidence interval (95%CI). Heterogeneity was detected by Cochran’s Q chi-square test and I2 analysis. P-values of less than 0.1 or I2 of more than 50% were considered to indicate heterogeneity. When heterogeneity existed, the random effect model was used to estimate the effect sizes; otherwise, the fixed model was administered. When heterogeneity was encountered, a sensitivity analysis was performed to investigate the influence of the removed study. Publication bias was measured by the Begg and Egger tests, with results presented by funnel plot. Significance was set at P<0.05. All analysis was conducted by REVMAN 5.2, and evidence recommendations and quality assessment were performed by GRADE version 3.6 [ 24].
Study selection
The initial search yielded 859 manuscripts. After extracting duplicates, 798 manuscripts remained, of which most were deemed irrelevant based on their title or/and abstract . Of the remaining 14 studies, four were not RCTs [14,15,25,26], two compared sorafenib with chemotherapeutic drugs [16,27], and two were subgroup analysis of prior clinical trials [12,28]. Ultimately, six studies were included in our analysis [8-11, 13,29] (Figure 3).
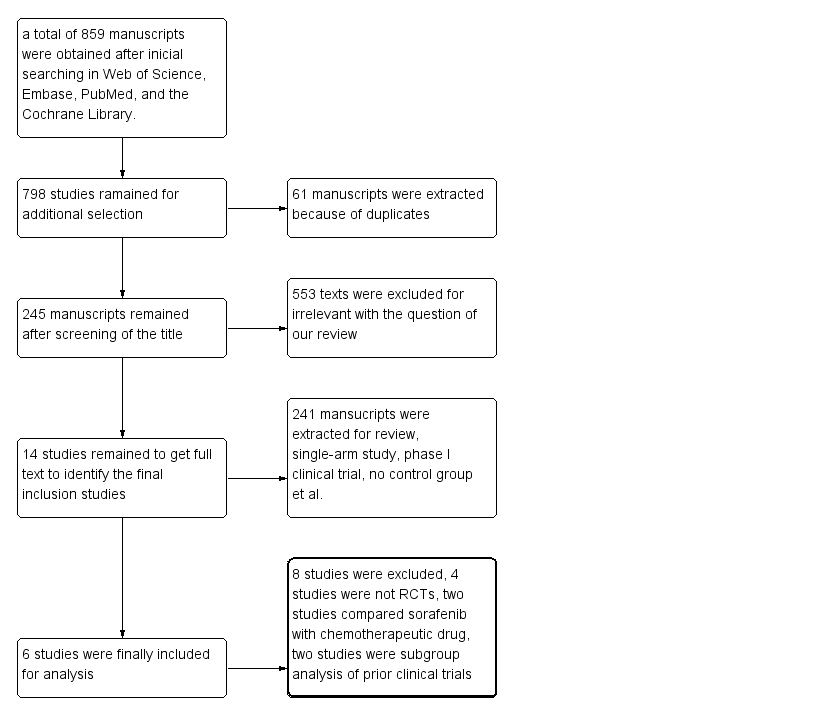
Figure 3. Flow gram of study selection
Baseline characteristics of included studies
A total of 1751 patients from Asia, Australasia, Europe, North America, Central America, and South America were enrolled in the six RCTs. Two studies compared sorafenib with placebo in the management of unresectable HCC, three studies compared the efficacy of sorafenib plus TACE with TACE alone in unresectable HCC, and sorafenib plus doxorubicin versus doxorubicin plus placebo were compared in one study. The number of subjects ranged from 62 to 602, and almost all patients presented a liver function of Child A and well performance status (PS 0-1). Two studies investigated the benefit of sorafenib in patients with BCLC B [13,29], and patients in the other four studies had BCLC B and BCLC C. Differences in the baseline characteristics did not reach significance among the enrolled studies (Table 1).
Table 1. Baseline characteristics of included studies
Reference |
Patients’ region |
Number |
Group |
Median age |
Male/female ratio(%) |
Child A ratio (%) |
ECOG PS(0-1)( %) |
BCLC stage |
Trial design |
Index |
[8] |
Europe and Australasia 87.4%,North America 9.3%,Central and South America 4.3% |
602 |
Sorafenib 299,
placebo 303 |
T: 64.9,
C: 66.3 |
T(87:13)
C(87:13) |
T:95%
C:98% |
T:92%
C:93% |
B:106
C:496 |
Sorafenib 400mg bid vs placebo bid |
Primary end points: OS, TTSP. secondary end points:TTRP, RR,DCR, safety |
[10] |
59.3%North America,34.3%Europe,2%Asia,
4.4%South America |
96 |
Doxorubicin+ sorafenib 47
vs doxorubicin + placebo 49 |
T:66
C: 65 |
T(66:34),
C(85.7:14.3) |
T:100,
C::95.9 |
T:85.1,
C:83.7 |
NR |
Doxorubicin every 21 days plus sorafenib 400mg bid vs doxorubicinplus placebo bid |
TTP, OS, PFS, response rate ,safety |
[11] |
Asia 100% |
458 |
TACE+sorafenib 229 vs
TACE+placebo 229 |
T:69
C:70 |
T(76:24)
C(73:27) |
T:100
C:100 |
T:100
C:100 |
NR |
After TACE, soarafenib 400mg bid vs placebo bid daily |
Primary end point: TTP secondary end point :OS, safety |
[9] |
Asia |
226 |
Sorafenib 150
Placebo 76 |
T:51
C:52 |
T(84.7:15.3)
C(66:34) |
T:97.3
C:97.4 |
T:94.7
C:96 |
B:10
C:216 |
Sorafenib 400mg bid vs placebo bid |
OS,TTP,TTSP,RR,
safety |
[13] |
Europe |
62 |
TACE+Sorafenib:31
TACE+placebo:31
|
T:73
C:72.8 |
T(58:42)
C(61:39) |
T:100
C:100 |
T:100
C:100 |
B:62 |
After TACE, Sorafenib 400mg bid vs placebo |
TTP, safety |
[2,9] |
Europe, Asia, and North America |
307 |
Sorafenib+TACE:154
Placebo+TACE:153 |
T:64.5
C:63.0 |
T(87.7:12.3)
C(82.4:17.6) |
T:100
C:100 |
T:100
C:100 |
B:307 |
Sorafenib 400mg bid+ TACE vs placebo+ TACE |
TTP,OS, DCR, safety |
Overall survival
Five studies reported overall survival, and we did not find significant heterogeneity among them(I2<50%, P>0.1) [8-11,29], so the fixed model was adopted for analysis. The pooled HR was 0.73(95%CI: 0.63, 0.85; P<0.001). The results demonstrated that sorafenib therapy can prolong overall survival of patients with unresectable HCC. The funnel plot detected no publication bias for this endpoint (Figure 4). We detected no publication bias in results of overall survival (Figure 5).
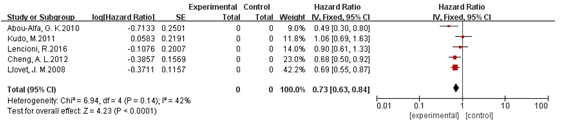
Figure 4. Analysis of overall survival
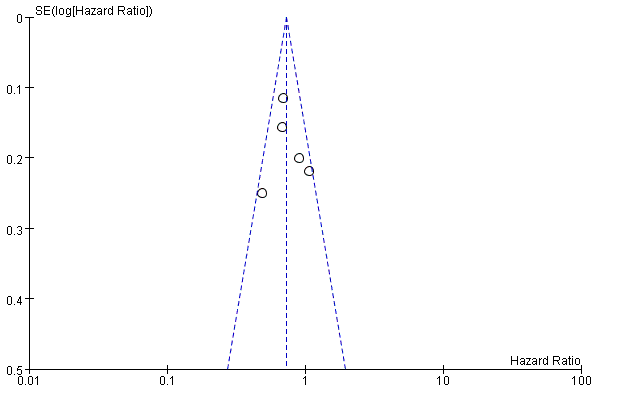
Figure 5. Detection of publication bias
Time to progression
Six studies provided data on time to progression [8-11,13,29]. We found significant heterogeneity among the studies (I2=89%, P<0.1), so a random model was used for effect size measurement. The pooled HR was 0.81 (95%CI: 0.55, 1.18; P>0.05). We found that sorafenib therapy did not benefit patients with unresectable HCC in terms of time to progression. Subsequently, we removed the study by Sansonno et al. [13], and a heterogeneity test of the remaining five studies yielded I2=58%, P=0.05<0.1. The pooled HR of these five studies was 0.67 (95%CI: 0.54, 0.83; P<0.05) (Figure 6 )
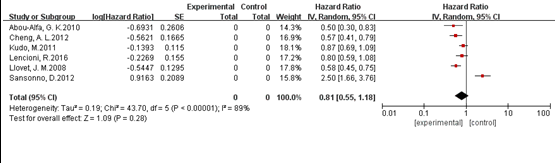
Figure 6. Analysis of time to progression
Disease control rate
Three studies reported the disease control rate [8,9,29]. Heterogeneity was not significant among these three studies (I2=49%, P>0.1). The pooled OR was 1.67 (95%CI: 1.14, 2.46; P<0.05), indicating that sorafenib therapy could improve tumor response in patients with unresectable HCC (Figure 7 )
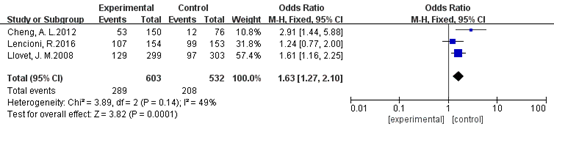
Figure 7. Analysis of disease control rate
Partial response
three studies published data on partial response [8, 9, 29]. No significant heterogeneity was found among these studies. A test for overall effect showed that the OR was 1.57 (95%CI: 0.95, 2.61; P>0.05). We found that sorafenib treatment did not increase partial response in patients with unresectable HCC (Figure 8).
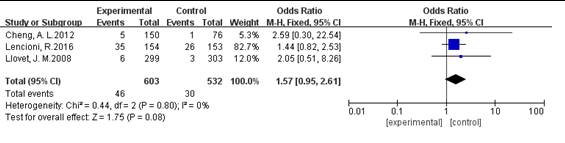
Figure 8. Analysis of partial response
Quality of evidence
According to the GRADE profile, evidence is divided into important and critical. The quality of evidence is ranked as high, moderate, low, and extremely low. Based on the instructions of the GRADE system, the result of our OS, TTP, DCR, and PR analysis was recommended as high quality evidence, see details in the following evidence profile of GRADE (Figure 1).
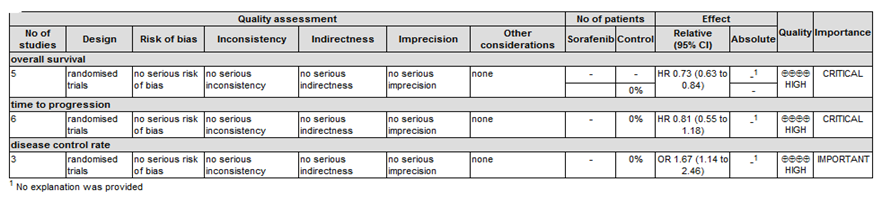
Figure 1. Evidence profile of GRADE
Adverse events
The most common adverse events of sorafenib therapy were hand-foot skin reaction (HFSR), fatigue, rash, hypertension, and diarrhea. All six studies reported information on adverse events, and investigators generally divided adverse events into all-grade and severe adverse events (grade 3 and 4) according to the National Cancer Institute’s Common Terminology Criteria for adverse events [30]. The RRs of HFSR, rash, hypertension, and diarrhea were 20.12(95%CI: 9.18, 44.08; P<0.05), 8.62(95%CI: 2.43, 30.60; P<0.05), 3.57(95%CI: 1.05, 12.04; P<0.05), and 4.06(95%CI: 2.27, 7.27; P<0.05), respectively. Therefore, administration of sorafenib could significantly increase the incidence of drug-related adverse events in patients with unresectable HCC.
This systematic review investigates the efficacy and safety of sorafenib for patients with unresectable HCC. We demonstrate that sorafenib therapy improves overall survival and increases the disease control rate for these patients. However, it did not lead to a significant difference in time to progression, partial response, or stable disease compared with control groups. In addition, sorafenib treatment significantly increased the incidence of HFSR, rash, hypertension, and diarrhea in patients with unresectable HCC.
Previous meta-analyses have obtained results consistent with those of the present study. Peng et al demonstrated that sorafenib improved overall survival (HR: 0.74) in patients with advanced HCC. They also found a time to progression benefit of sorafenib therapy (HR: 0.69; P<0.05), which was inconsistent with our finding (HR:0.81;P>0.05) [18]. That study included seven RCTs with a total of 3807 patients, of which, two clinical trials compared the efficacy of sorafenib with other systemic drugs [16, 27], decreasing the reliability of the findings. In the present review, when we removed the study by Sansonno [13], time to progression in the sorafenib therapy group was significantly longer than that of the control group(HR:0.67;95%CI: 0.54, 0.83; P<0.05). The sample size of the Sansonno study was relatively small, which might have led to selection bias in the results. Moreover, etiology was more heterogeneous in this study compared with the other five studies. Patients with underlying hepatitis C virus (HCV) induced BCLC B HCC were included in the trial, and investigators found that sorafenib plus TACE provided superior benefit for patients with BCLC B HCC in terms of time to progression. Previous studies demonstrated that HCC patients with underlying HCV infection had higher long-term recurrence and multi-center progression [31]. Sorafenib may delay tumor progression by suppressing both tumor growth and neoangiogenesis, and decreasing the rate of metachronous multi-center progression [31]. Thus, the HR of time to progression (HR=2.5) was higher in the Sansonno study than that in the other five studies.
Three prior systematic reviews compared the effect of sorafenib plus TACE with TACE alone in patients with advanced HCC [17, 20, 21]. They all found that sorafenib combined with TACE significantly prolonged the time to progression, but did not significantly improve overall survival in patients with advanced HCC, which was inconsistent with our findings. As we know, sorafenib decreases short and long term recurrence and tumor progression, and time to progression is an index influenced by multiple tumor progression and metachronous [32,33]. In addition, TACE is regarded as a regional therapy recommended for patients with intermediate HCC, which can induce satisfactory tumor necrosis when the diameter<5cm; embolism could decrease the blood supply to the target tumor, further slowing the disease progression [34]. Thus, patients with sorafenib combined with TACE therapy may have a superior benefit in time to progression than those with TACE or sorafenib alone.
The incidence of severe drug-related adverse events was higher in the sorafenib treatment group than in the control group, which was consistent with the findings of previous reviews. The most common drug-related adverse events of sorafenib are HFSR, rash, diarrhea, hypertension, and fatigue. Other rare severe adverse events were reported in previous studies, but in our review, none of the six included studies reported fatal adverse events. Previous reviews even suggested that early dermatologic adverse events predicted better survival of patients treated with sorafenib [35]; however, more studies are needed to reach a conclusion. Our review demonstrated that sorafenib treatment could raise the incidence of adverse events, but that risk is fairly tolerable. In clinical practice, investigators have proposed measures to mitigate these events, which could increase the safety of sorafenib management [36].
Our study has some limitations. First, the number of enrolled patients was somewhat small, so while the included studies were all RCTs, the number of subjects decreases the reliability of the final conclusion. Second, disease etiology, age, BCLC stage, and PS grade are all prognostic factors for unresectable HCC., but we did not perform subgroup analysis to explore the influence of these factors in sorafenib treatment.
We conclude that sorafenib can benefit patients with unresectable HCC in overall survival and disease control , which increasing the incidence of drug-related adverse events, which are comparatively tolerable. The future frontier may lie in the investigation of sorafenib for management of intermediate HCC, but more RCTs with large sample size are needed to explore sorafenib for the management of unresectable HCC.
Xu MQ designed the review; Yi PS wrote the paper; Xu LL discussed the theme presented in article, Zhang M and Xu MQ revised the draft. Peng Sheng Yi and Min Huang contributed equally to this work.
- Torrecilla S, Llovet JM (2015) New molecular therapies for hepatocellular carcinoma. Clin Res Hepatol Gastroenterol 39 Suppl 1: S80-85. [Crossref]
- Song P, Feng X, Zhang K, Song T, Ma K, et al. (2013) Screening for and surveillance of high-risk patients with HBV-related chronic liver disease: promoting the early detection of hepatocellular carcinoma in China. Biosci Trends 7:1-6. [Crossref]
- Su C, Lin Y, Niu J, , Cai L (2014) Association between polymorphisms in tumor suppressor genes and oncogenes and risk of hepatocellular carcinoma: a case-control study in an HCC epidemic area within the Han Chinese population. Med Oncol 31: 356. [Crossref]
- Wang D, Cai H, Yu WB, Yu L (2014) Identification of hepatitis B virus X gene variants between hepatocellular carcinoma tissues and pericarcinoma liver tissues in Eastern China. Int J Clin Exp Pathol 7:5988-96. [Crossref]
- Cillo U, Vitale A, Grigoletto F, Farinati F, Brolese A, et al. (2006) Prospective validation of the Barcelona Clinic Liver Cancer staging system. J Hepatol 44: 723-731. [Crossref]
- Mazzanti R, Arena U, Tassi R (2016) Hepatocellular carcinoma: Where are we? World J Exp Med 6: 21-36. [Crossref]
- Xu L, Gao H, Huang J, Wang H, Zhou Z, et al. (2015) Antiviral therapy in the improvement of survival of patients with hepatitis B virus-related hepatocellular carcinoma treated with sorafenib. J Gastroenterol Hepatol 30: 1032-9. [Crossref]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390. [Crossref]
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, et al. (2009) Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol 10: 25-34. [Crossref]
- Abou-Alfa GK, Johnson P, Knox JJ, Capanu M, Davidenko I, et al. (2010) Doxorubicin plus sorafenib vs doxorubicin alone in patients with advanced hepatocellular carcinoma: a randomized trial. JAMA 304: 2154-2160. [Crossref]
- Kudo M, Imanaka K, Chida N, Nakachi K, Tak WY, et al. (2011) Phase III study of sorafenib after transarterial chemoembolisation in Japanese and Korean patients with unresectable hepatocellular carcinoma. Eur J Cancer 47:2117-27. [Crossref]
- Bruix J, Raoul JL, Sherman M, Mazzaferro V, Bolondi L, et al. (2012) Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma: subanalyses of a phase III trial. J Hepatol 57: 821-829. [Crossref]
- Sansonno D, Lauletta G, Russi S, et al. Transarterial chemoembolization plus sorafenib: a sequential therapeutic scheme for HCV-related intermediate-stage hepatocellular carcinoma: a randomized clinical trial. Oncologist 2012;17:359-66. [Crossref]
- Abdel-Rahman O, Abdel-Wahab M, Shaker M, et al. Sorafenib versus capecitabine in the management of advanced hepatocellular carcinoma. Med Oncol 2013;30:655. [Crossref]
- Bai W, Wang YJ, Zhao Y, et al. Sorafenib in combination with transarterial chemoembolization improves the survival of patients with unresectable hepatocellular carcinoma: a propensity score matching study. J Dig Dis 2013;14:181-90. [Crossref]
- 16. Johnson PJ, Qin S, Park JW, et al. Brivanib versus sorafenib as first-line therapy in patients with unresectable, advanced hepatocellular carcinoma: results from the randomized phase III BRISK-FL study. J Clin Oncol 2013;31:3517-24. [Crossref]
- Liu L, Chen H, Wang M, Zhao Y, Cai G, et al. (2014) Combination therapy of sorafenib and TACE for unresectable HCC: a systematic review and meta-analysis. PLoS One 9: e91124. [Crossref]
- Peng S, Zhao Y, Xu F, Jia C, Xu Y, et al. (2014) An updated meta-analysis of randomized controlled trials assessing the effect of sorafenib in advanced hepatocellular carcinoma. PLoS One 9: e112530. [Crossref]
- Shao YY, Shau WY, Chan SY, et al. Treatment efficacy differences of sorafenib for advanced hepatocellular carcinoma: a meta-analysis of randomized clinical trials. Oncology 2015;88:345-52. [Crossref]
- Hu MD, Jia LH, Liu HB, Zhang KH, Guo GH (2016) Sorafenib in combination with transarterial chemoembolization for hepatocellular carcinoma: a meta-analysis. Eur Rev Med Pharmacol Sci 20: 64-74. [Crossref]
- Wang G, Liu Y, Zhou SF, et al. Sorafenib combined with transarterial chemoembolization in patients with hepatocellular carcinoma: a meta-analysis and systematic review. Hepatol Int 2016. [Crossref]
- Furlan AD, Pennick V, Bombardier C, van Tulder M; Editorial Board, Cochrane Back Review Group (2009) 2009 updated method guidelines for systematic reviews in the Cochrane Back Review Group. Spine (Phila Pa 1976) 34: 1929-1941. [Crossref]
- Eisenhauer EA1, Therasse P, Bogaerts J, Schwartz LH, Sargent D, et al. (2009) New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45: 228-247. [Crossref]
- Atkins D, Best D, Briss PA, Eccles M, Falck-Ytter Y, et al. (2004) Grading quality of evidence and strength of recommendations. BMJ 328: 1490. [Crossref]
- Hu H, Duan Z, Long X, Hertzanu Y, Shi H, et al. (2014) Sorafenib combined with transarterial chemoembolization versus transarterial chemoembolization alone for advanced-stage hepatocellular carcinoma: a propensity score matching study. PLoS One 9: e96620. [Crossref]
- Erhardt A, Kolligs F, Dollinger M, Schott E, Wege H, et al. (2014) TACE plus sorafenib for the treatment of hepatocellular carcinoma: results of the multicenter, phase II SOCRATES trial. Cancer Chemother Pharmacol 74: 947-54. [Crossref]
- Cheng AL, Kang YK, Lin DY, Park JW, Kudo M, et al. (2013) Sunitinib versus sorafenib in advanced hepatocellular cancer: results of a randomized phase III trial. J Clin Oncol 31: 4067-4075. [Crossref]
- Cheng AL, Guan Z, Chen Z, Tsao CJ, Qin S, et al. (2012) Efficacy and safety of sorafenib in patients with advanced hepatocellular carcinoma according to baseline status: subset analyses of the phase III Sorafenib Asia-Pacific trial. Eur J Cancer 48: 1452-65. [Crossref]
- Lencioni R, Llovet JM, Han G, Tak WY, Yang J, et al. (2016) Sorafenib or placebo plus TACE with doxorubicin-eluting beads for intermediate stage HCC: The SPACE trial. J Hepatol 64: 1090-1098. [Crossref]
- Dueck AC, Mendoza TR, Mitchell SA, et al. Validity and Reliability of the US National Cancer Institute's Patient-Reported Outcomes Version of the Common Terminology Criteria for Adverse Events (PRO-CTCAE). JAMA Oncol 2015;1:1051-9. [Crossref]
- Sasaki Y, Yamada T, Tanaka H, Ohigashi H, Eguchi H, et al. (2006) Risk of recurrence in a long-term follow-up after surgery in 417 patients with hepatitis B- or hepatitis C-related hepatocellular carcinoma. Ann Surg 244: 771-780. [Crossref]
- Prajapati HJ, Xing M, Spivey JR, Hanish SI, El-Rayes BF, et al. (2014) Survival, efficacy, and safety of small versus large doxorubicin drug-eluting beads TACE chemoembolization in patients with unresectable HCC. AJR Am J Roentgenol 203: W706-714. [Crossref]
- Xing M, Kokabi N, Prajapati HJ, Close O, Ludwig JM, et al. (2016) Survival in unresectable AJCC stage I and II HCC and the effect of DEB-TACE: SEER versus tertiary cancer center cohort study. J Comp Eff Res 5: 141-154. [Crossref]
- Tsurusaki M, Murakami T (2015) Surgical and Locoregional Therapy of HCC: TACE. Liver Cancer 4: 165-175. [Crossref]
- Reig M, Torres F, Rodriguez-Lope C, Forner A, LLarch N, et al. (2014) Early dermatologic adverse events predict better outcome in HCC patients treated with sorafenib. J Hepatol 61: 318-324. [Crossref]
- Schott E, Ebert MP, Trojan J (2012) Treatment of hepatocellular carcinoma with sorafenib - focus on special populations and adverse event management. Z Gastroenterol 50: 1018-1027. [Crossref]








