Abstract
Silver nanoparticles/silver chloride (Ag/AgCl) biogenically synthesized acted efficiently on all the bacteria that produced beta-lactamases (Extended Spectrum beta-lactamase-ESBL or Klebsiella pneumoniae carbapenemase-KPC). The presence of imipenem (IPM)/Ag/AgCl showed synergism between the IPM antibiotic and Ag/AgCl nanoparticles. The results obtained with E. coli wild type and beta-lactamases producing bacteria reinforced the potentiality of silver nanoparticles on beta-lactamase enzymes, since E. coli is free of any beta-lactamase enzymatic mechanism, and it was not observed any alteration in the IPM zone inhibition with the silver nanoparticles. The study of biogenic nanoparticles efficacy against resistant microorganism is very important due to progressive increase of antibiotic resistant bacteria.
Key words
Enterobacteriaceae, extended spectrum beta-lactamase, KPC, silver nanoparticles, imipenem
Introduction
Klebsiella pneumoniae carbapenemase (KPC)-producing bacteria are a group of emerging highly drug-resistant Gram-negative bacilli causing infections associated with significant morbidity and mortality. Although there are new agents within existing classes of antimicrobials, currently there are no new classes of antimicrobials in the later phases of development with activity against multi drug resistant Gram-negative bacteria [1].
The worldwide spread of KPC-producing K. pneumoniae strains (KPC-KP) has revealed the successful dissemination of a major clone defined as sequence type 258 (ST258). Since 2006, KPC-KP has arisen in South America, particularly in countries bordering Uruguay, Argentina and Brazil [2].
The enzymes beta-lactamases are distributed in the planet and have the capacity of degrade the beta-lactamic antibiotics, a class of pharmaceutical widely used in a severe bacterial infection. Among these enzymes is the ESBL (Extended Spectrum beta-lactamase) and KPC (Klebsiella pneumoniae carbapenemase) that exhibited different action spectra on beta-lactamic antibiotics [3-5]. Many efforts in these last years have dedicated in the development of new drugs or materials due to the emergence and increase of microbial organisms resistant to multiple antibiotics. The most new promising nanomaterials with antibacterial properties are the metallic nanoparticles such as silver nanoparticles [6]. Silver nanoparticles are from a long time known for many researchers, and in this direction were published excellent reviews in the last two years [7-15]. Few reviews were reported on biogenic AgCl nanoparticles [16].
Since, silver nanoparticles as also AgCl nanoparticles showed excellent activity against bacteria, it was interesting to evaluate the affectivity against resistant bacteria.
Then, the aim of this work was to evaluate the antibacterial activities of Ag/AgCl nanoparticles combined with imipenem (IPM) against bacteria with resistance mechanism for beta-lactamase.
Experimental part
Biosynthesis of silver nanoparticles: The Fusarium oxysporum (F. oxysporum) strain used was the following: 07 SD, from ESALQ-USP Genetic and Molecular Biology Laboratory-Piracicaba, S.P., Brazil. The fungal inoculates were prepared in a malt extract 2% and yeast extract 0.5% at 28°C in Petri plates. The liquid fungal growth was carried out in the presence of yeast extract 0.5% at 28°C for 6 days. The biomass was filtrated and resuspended in sterile water. Approximately 10 g of F. oxysporum biomass was taken in a conical flask containing 100 mL of distilled water, kept for 72 h at 28°C and then the aqueous solution components were separated by filtration and/or centrifugation. To this solution, AgNO3 (10-2 M) was added and kept for 72 hours at 28°C [17].
Characterization: It is known that the absorption peak shifts toward higher energy with a decrease in the size of silver nanoparticles mycofabricated. Absorbance spectrum of colloidal samples was taken from 200 to 800 nm, by UV-Vis spectrometer (Perkin Elmer, Lambda-950). The absorption spectrum showed a Plasmon resonance absorbance around 420-440 nm. The crystal structure of silver nanoparticles was characterized by X-ray diffraction (XRD). The measurements were carried out on an X-ray diffractometer (Shimadzu XRD 7000). The patterns with Cu Kα radiation (λ=0.15406 nm) at a 40 kV voltage, 30 mA current with a 2° min-1 scanning rate were recorded in the 5°-90° 2θ region. The nanoparticle dispersion was diluted with deionized water (1:10 v/v), then the mean diameter (Z-average) and zeta potential were measured by the technique of Dynamic Light Scattering (DLS) and Electrophoretic Mobility respectively, using the equipment Nano ZS Malvern ZetaSizer with fixed angle of 173° at 25°C. The morphology and particle size of the silver nanoparticles were investigated in a Carl Zeiss Libra 120 Plus (with a -filter in column) transmission electron microscope, operated at an 80 kV acceleration voltage and using a tungsten thermionic source. An Olympus camera with iTEM software was used for image.
Antibacterial assays: It was studied 5 strains with resistance mechanisms for beta-lactamases (1 SER: Serratia mascescens producer of carbapenemase-KPC; 3 KPN: Klebsiella pneumoniae producer of carbapenemase-KPC confirmed through molecular biology technique blaKPC gene; 1 ESBL: Klebsiella pneumoniae ATCC 700603 producer of Extended Spectrum beta-lactamase) and a negative control (Escherichia coli ATCC 25922). The ESBL beta-lactamase phenotype it was carried out by combined disc [18].
The KPC carbapenemase was assessed with two different phenotypic detection methods: A modified Hodge test (MHT) [19], and boronic assay [20]. The latter was considered positive when the difference of inhibition zone between carbapenem disk with boronic acid and the carbapenem alone was equal or higher than 5 mm [21].
Initially, it was determined the minimum inhibitory concentration (MIC) by macrodilution [22] of silver nanoparticles (from 84.5 to 1.32 µg/mL). The MIC value was used in the diffusion disk assay. In the diffusion disk assay, the agar Mueller Hinton (Oxoid, United Kingdom) plates were inoculated with each bacteria adjusted to standard equivalent to 0.5 McFarland (1.5 x 108 UFC mL-1). It was used four disks of imipenem 30 µg (IPM) and on two of these disks were applied 6.2 µL (10.52 µg disk-1) of silver nanoparticles solution of 1690 µg mL-1. In parallel, two blank disks of filter paper received 6.2 µL (10.52 µg disk-1) of silver nananoparticles solution of 1690 µg mL-1 (control). As the plates were incubated for 18 h at 35°C ± 2°C and the inhibition diameter average was registered. All the assays were carried out in duplicate.
Results and discussions
Biosynthesis of silver nanoparticles: The formation of Ag/AgCl was investigated by UV-Vis spectroscopy technique. In Figure 1a shows the presence of a plasmon band around 429 nm is attributed to the formation of pseudo-spherical silver nanoparticles and corresponds to the surface resonance. In Figure 1b of XRD pattern is shown typically peaks at 38.1°, 43.8°, 64.2°, 77.2° e 81.5°, corresponding to the (111), (200), (220), (311) and (222) diffractions for face centered cubic (fcc) silver phase (JCPS 04-0783), that coexists with the cubic phase of AgCl at 27.9°, 32.3°, 46.3°, 55.0°, 57.6°, 67.6°, 74.6°, 76.9°, and 85.7° and that corresponds to the (111), (200), (220), (311), (222), (400), (331), (420), and (422) planes (JCPDS file: 31-1238) in a similar way as recently published by laccase Ag/AgCl nanoparticles synthesis [8,16] or from F. oxysporum [23].
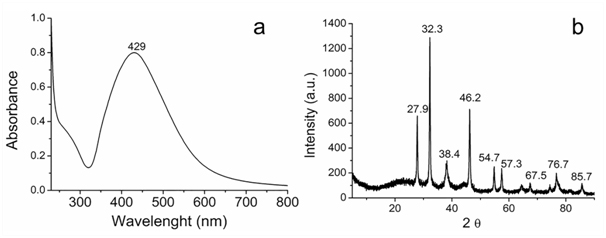
Figure 1. (a) UV-vis spectrum and (b) XRD diffraction patterns of the Ag/AgCl.
The zeta potential for the Ag/AgCl was evaluated to estimate the stability for aggregation of the nanoparticles. The zeta potential absolute value can be usually used as an indicator of a colloidal system stability. The zeta potential value for the Ag@AgCl was found to be approximately – 26.5 mV, which is, according to literature [24], features state stability. Suggesting the stability of the Ag/AgCl occurs also due to the presence of molecules adsorbed on surface, formed during biosynthesis.
The morphology, size and size distribution of silver nanoparticles was investigated by TEM. Figure 2 shows TEM images obtained for the silver nanoparticles pseudo-spherical morphology. It was observed aggregation of silver nanoparticles, which can be associated with the drying process for the preparation of the sample for TEM analysis. It can be also observed the presence of Ag/AgCl nanoparticles with an average size of 4 nm. The obtained Ag/AgCl nanoparticles have a size distribution with a mean diameter of 55 ± 18 nm, from the TEM image (The total number of particles counted for the histogram was 198). This, it was different from DLS measurement, since in that case the hydrodynamic radius was measured that as it is known protein capped the silver nanoparticles (89 nm with a zeta potential of -26.5 mV) [17,25,26], and showing excellent antibacterial activities [27-29].

Figure 1. (a) UV-vis spectrum and (b) XRD diffraction patterns of the Ag/AgCl.
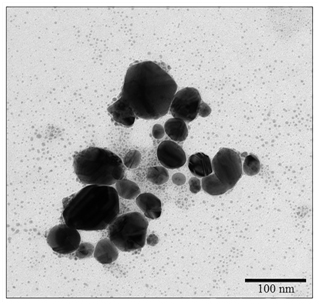
Figure 2. TEM micrograph of A/AgCl nanoparticles.
Antibacterial assay: The disk diffusion test results showed very similar values between the different treated bacteria, since for E. coli ATCC 25922 (no beta-lactamase ESBL or KPC resistance mechanisms) had substantially equivalent zones inhibition (Table 1). This suggests that the mechanism of action of Ag/AgCl nanoparticles is similar to those different bacteria and independent of the presence of enzymatic mechanisms of resistance to beta-lactamase. This finding was confirmed by the MIC determination, since all bacterial strains presented the same profile sensitivity (Minimum Inhibitory Concentration=10.52 µg/mL of Ag/AgCl nanoparticles).
Strain |
IPM
Zone diameter average (mm)
(a) |
IPM/Ag/AgCl
Zone diameter average (mm)
(b) |
Ag/AgCl
Zone diamenter average (mm)
(c) |
SER* |
17.0 ± 0 |
18.5 ± 0.5 |
7.5 ± 0.5 |
KPN 710* |
19.0 ± 0 |
20.0 ± 0 |
7.0 ± 0 |
KPN PCR* |
18.0 ± 0 |
19.0 ± 0 |
6.7 ± 0.25 |
KPN HC PCR* |
18.0 ± 0 |
18.5 ± 0.5 |
6.0 ± 0 |
ESBL 700603 |
28.0 ± 0 |
29.0 ± 0 |
7.5 ± 0.5 |
E. coli 25922 |
30.0 ± 0 |
30.0 ± 0 |
7.0 ± 0 |
Table 1. Evaluation of synergistic effect of 10.52 µg/disk silver nanoparticles (Ag/AgCl) with imipenem (IPM).
SER: Serratia mascescens; KPN: Klebsiella pneumoniae; ESBL: K. pneumoniae producer of Extended Spectrum Beta-Lactamase; *: KPC producing; NSE: Non synergistic effect; IPM: imipenem; Ag/AgCl: silver nanoparticles.
However, all the bacteria that produced beta-lactamases (ESBL or KPC) demonstrated an increase of inhibition zone diameter with the presence of IPM/silver nanoparticles when compared to the diameter produced only with IPM. This behavior suggests a synergism between the IPM antibiotic and Ag/AgCl nanoparticles. The results obtained with E. coli 25922 and other bacteria reinforced the potentiality of silver nanoparticles on beta-lactamase enzymes, since E. coli is free of any beta-lactamase enzymatic mechanism, and it was not observed any alteration in the IPM zone inhibition with the silver nanoparticles. Therefore, it is evident that the action of imipenem (IPM) on Enterobacteriaceae producing beta-lactamase was incresead due the nanoparticles.
Final remarks: Due to the severity of the action of resistant bacteria is essential to search for new antibiotics. Recently e.g. two patients who were admitted to the Intensive Care Unit (ICU) of the Emergency Sergipe Hospital (Huse) (Brazil) died in the and according to the manager of the Hospital Infection Control, patients were infected with bacteria resistant KPC [30]. Then, our results with Ag/AgCl nanoparticles alone or their formulations in combination with commonly used antibiotics could be used as effective bactericidal agents showing to be an interesting nanomaterial against resistant bacteria.
Acknowledgements
Support from FAPESP, CNPq, INOMAT (MCTI/CNPq), Brazilian Network of Nanotoxicology (MCTI/CNPq) and NANOBIOSS (MCTI/CNPq) is gratefully acknowledged.
References
- Arnold RS, Thom KA, Sharma S, Phillips M, Kristie Johnson J, et al. (2011) Emergence of Klebsiella pneumoniae carbapenemase-producing bacteria. South Med J 104: 40-45. [Crossref]
- Marquez C, Ingold A, Echeverría N, Acevedo A, Vignoli R, et al. (2014) Emergence of KPC-producing Klebsiella pneumoniae in Uruguay: infection control and molecular characterization. New Microbes New Infect 2: 58-63. [Crossref]
- Bratu S, Mooty M, Nichani S, Landman D, Gullans C, et al. (2005) Emergence of KPC-possessing Klebsiella pneumoniae in Brooklyn, New York: epidemiology and recommendations for detection. Antimicrob Agents Chemother 49: 3018-3020. [Crossref]
2021 Copyright OAT. All rights reserv
- Queenan AM, Bush K (2007) Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev 20: 440-458, table of contents. [Crossref]
- Meyer G, Picoli SU (2011) Phenotypes of beta-lactamases in Klebsiella pneumoniae from emergency hospital of Porto Alegre. J Bras Patol Med Lab. 47: 25-31.
- DuránN, Durán M, de Jesus MB, FávaroWJ, et al. (2016) Antimicrobial activity mechanisms of silver nanoparticles: An overview. Nanomedicine:NBM. 12:789–799 (2016).
- Durán N, Seabra AB, De Lima R (2014a) Cytotoxicity and genotoxicity of biogenically synthesized silver nanoparticles. In Nanotoxicology: Materials, methodologies, and assessments. (N. Durán, S.S. Guterres, O.L. Alves, Eds). Springer 11:245-263.
- Durán M, Silveira CP, Durán N (2015) Catalytic role of traditional enzymes for biosynthesis of biogenic metallic nanoparticles: a mini-review. IET Nanobiotechnol 9: 314-323. [Crossref]
- Abbasi E, Milani M,Fekri Aval S, Kouhi M, et al. (2016) Silver nanoparticles: Synthesis methods, bio-applications and properties. Crit Rev Microbiol 42: 173-180. [Crossref]
- Faramarzi MA,Sadighi A (2013) Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv Colloid Interface Sci 189-190: 1-20. [Crossref]
- Mittal J, Batra A, Singh A, Sharma MM (2014) Phytofabrication of nanoparticles through plant as nanofactories. Adv Nat Sci Nanosci Nanotechnol 5: 043002.
- Mashwani Z, Khan T, Khan MA, Nadhman A (2015) Synthesis in plants and plant extracts of silver nanoparticles with potent antimicrobial properties: Current status and future prospects. Appl Microbiol Biotechnol 99:9923–9934.
- Natsuki J, Natsuki T, Hashimoto Y (2015) A Review of silver nanoparticles: Synthesis methods, properties and applications. Inter J Mat Sci Appl 4: 325-332.
- Moghaddam AB, Namvar F, Moniri M, Tahir PMd, et al. (2015) Nanoparticles biosynthesized by fungi and yeast: A review of their preparation, properties, and medical applications. Molecules 20
- Keat CL, Aziz A, Eid AM, Elmarzugi NA (2015) Biosynthesis of nanoparticles and silver nanoparticles. Bioresour Bioprocess 2:47.
- Durán N, Cuevas R, Cordi L, Rubilar O, et al. (2014b) Biogenic silver nanoparticles associated with silver chloride nanoparticles (Ag@AgCl) produced by laccase from Trametes versicolor. Springer Plus 3: 645
- Durán N, Marcato PD, Alves OL, Souza GI, Esposito E (2005) Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnology 3: 8. [Crossref]
- CLSI-Clinical and laboratory Standards Institute. Performance standards for antimicrobial susceptibility testing. CLSI document M100-S21. Wayne, Pennsylvania, USA.
- Amjad A, Mirza Ia, Abbasi S, Farwa U, Malik N, et al. (2011) Modified Hodge test: A simple and effective test for detection of carbapenemase production. Iran J Microbiol 3: 189-193. [Crossref]
- van Dijk K, Voets GM, Scharringa J, Voskuil S, Fluit AC, et al. (2014) A disc diffusion assay for detection of class A, B and OXA-48 carbapenemases in Enterobacteriaceae using phenyl boronic acid, dipicolinic acid and temocillin. Clin Microbiol Infect 20: 345-349. [Crossref]
- Doi Y, Potoski BA, Adams-Haduch JM, Sidjabat HE, Pasculle AW, et al. (2008) Simple disk-based method for detection of Klebsiella pneumoniae carbapenemase-type beta-lactamase by use of a boronic acid compound. J Clin Microbiol 46: 4083-4086. [Crossref]
- Manfredini C, Picoli SU, Becker AP (2011) Comparison of methods to determination of vancomycin sensivity in methicillin-resistant Staphylococcus aureus. J Bras Patol Med Lab 47:141-45.
- Andrade PF, Nakazato G, Durán N (2016) Antibacterial properties of carbon dots extracted from soluble coffe. Nanomedicine: NBM.
- Everett DH (1988) Basic principles of colloid science. The Royal Society of Chemistry, London,127-145.
- Durán N, Marcato PD, Souza G, Alves, OL, et al. (2007) Antibacterial effect of silver nanoparticles produced by fungal process on textile fabrics and their effluent treatment. J Biomed Nanotechnol 3: 203-208.
- Durán N, Silveira CP, Durán M, et al. (2015) Silver nanoparticle protein corona and toxicity: a mini-review. J Nanobiotechnology 13: 55. [Crossref]
- Durán N, Marcato PD (2012) Biotechnological routes to metallic nanoparticles production: mechanistics aspects, antimicrobial activity, toxicity and industrial applications. In Nano-Antimicrobials: Progress and Prospects (M. Rai and N. Cioffi, Eds).Springer, Germany: 337-374.
- Rai M, Kon K, Ingle A, Duran N, Galdiero S, et al. (2014) Broad-spectrum bioactivities of silver nanoparticles: the emerging trends and future prospects. Appl Microbiol Biotechnol 98: 1951-1961. [Crossref]
- RaiM, BirlaS, Gupta I, Ingle A, et al.(2014b) Nanosilver: an inorganic nanoparticle with myriad potential applications. Nanotechnol Rev 3: 281–309.
- Globo (2015) Two patients who were admitted to the Intensive Care Unit (ICU) of the Emergency Sergipe Hospital. Brazil.


