Abstract
Nowadays, sentinel diagnostic is been made with technetium-99m-(Tc-99m)-nanocolloide as a radioactive marker and sometimes patent blue. In the last years, indocyanin green has been evaluated for sentinel diagnostic in different tumor entities. Indocyane greene is a fluorescent molecule which emits a light signal in the near infrared band after excitation. Our study aims to evaluate indocyanin green compared to the gold standard Tc-99m-nanocolloid.
We report over a patient with an unifocal vaginal cancer. Sentinel diagnostic was carried out using Tc-99m-nanocolloid, indocyanin green and patent blue. After SPECT/CT, we examined each groin for light signals from the near infrared band, for radioactivity and for blue staining. A sentinel lymph node was defined as a Tc-99m-nanocolloide positive lymph node. All sentinel lymph nodes and all additional blue or fluorescent lymph nodes were excised and tested; then sent to histologic examination.
The procedure could be accomplished without problems. We found four sentinel lymph nodes with indocyanin green fluorescence, which were positive for radioactivity from Tc-99m and therefore defined as sentinel lymph nodes. Two further lymph nodes, which showed positivity for indocyanin green signals, but not for Tc-99m were found in the left groin. The final tumor stage was pT1b, pN0 (0/6, 0/4 SLN). We saw no adverse events or complications in this patient.
Our case report shows that near infrared fluorescence deriving from ICG is a promising approach for sentinel identification in vaginal cancer. If studies using sentinel lymphonodectomy will be planned, a combined approach using Tc-99m-nanocolloide and indocyanin green could be useful.
Key words
indocyanine green, sentinel, technetium-99m-nanocolloide, vaginal cancer
Introduction
Vaginal cancer is a very rare malignant disease with an incidence of 0.4 – 0.5 per 100,000 women per year. The therapeutic approach in vaginal cancer depends on the tumor stage and localization and may include a local radical excision, partial or total colpectomy with paracolpic tissue +/- radical hysterectomy or radiochemotherapy and / or vaginal brachytherapy [1-4]. The most important prognostic parameter in vaginal cancer is the lymphatic status of the draining lymph nodes [5-7]. In vaginal cancer, the lymphatic drainage pathways have a great variance and are depending on the tumor site in the vagina [7,8]. As a basic rule, a tumor localized in the lower third of the vagina is likely to drain in the inguinal lymphnodes like in vulvar cancer, whereas a tumor in the upper third is likely to drain into the pelvic lymphnodes like in cervical cancer.
The SLN technique in gynecologic oncology is nowadays often used in cervical and vulvar cancer [9-13]. As a complete groin dissection is associated with high morbidity like particularly lymphoceles and lymph edema, wound healing disorders and infections, sentinel lymph node (SLN) technique has been shown to reduce theses significantly in vulvar cancer [9,10].
The SLN technique may lead to an optimized therapy of patients with vaginal cancer and has been successfully applied using radioactive markers [7,14,15]. After a complete inguinofemoral or pelvic lymph node dissection, the same morbidity as in vulvar or cervical cancer patients is to expect. However, the prognosis of a nodal recurrence is poor and associated with a high mortality in vulvar and cervical cancer [11,16]. The gold standard of sentinel mapping in vulvar or cervical cancer is the use of radioactive technetium-99m-nanocolloide (Tc-99m).
Near infrared fluorescence by indocyanine green (ICG) has been shown to be a promising approach for a successful SLN and resection in different gynecologic tumors [17-19]. After subcutaneous or intracutaneous injection, ICG is drained by lymphatic vessels. ICG is a fluorescence dye which has to be excited in the near infrared band at a wavelength of 807 nm and then emits a light signal of 822 nm. We present the first case report of a SLN detection using ICG in vaginal cancer.
Patient and methods
The study protocol for using ICG in different gynecologic cancers including vaginal cancer was reviewed by the ethical committee of our institution. Written informed consent was obtained from the patient reported. The patient was a 58 year old woman with a histologically proven vaginal cancer in stage FIGO I. The tumor was located at the lower vaginal third in the rear wall with a diameter of 2 cm for the invasive lesion. The groins and pelvic lymph nodes were without suspicion of tumor infiltration by palpation, sonography and computer tomography.
A radical partial colpectomy of the lower vaginal third with bilateral inguinal and / or pelvic sentinel lymponodectomy, depending on the sentinel drainage in her case, was discussed and scheduled with the patient. Following our standard protocol for sentinel diagnostic in vulva, vaginal and cervical cancer, 10 megabecquerel (MBq) of Tc-99m-nanocolloide in 4 doses of 1 ml sterile water were injected intradermally at 4 points around the tumor site 3 hours prior to surgery. After this procedure, we performed a single photon emission computed tomography combined with conventional tomography (SPECT/CT). At this, we saw a lymphatic drainage into two inguinal SLN (Figure 1).
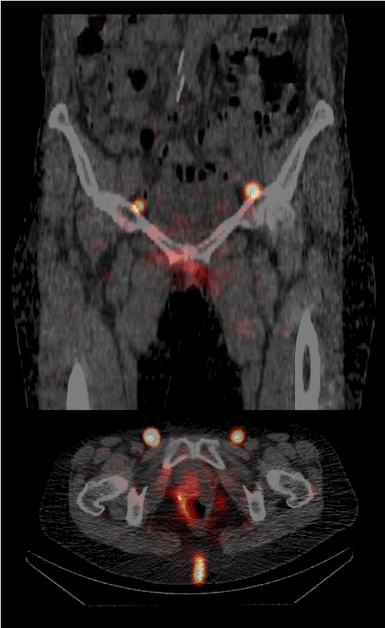
Figure 1. Single photon emission computed tomography combined with conventional tomography (SPECT/CT) from the patient showing the two inguinal sentinel sites; top: sagittal plane; bottom: transverse plane.
We dissolved a dose of 25 mg ICG (ICG Pulsion, Pulsion Medical Systems SE, Feldkirchen, Germany) in 20 ml sterile water. This leads to a concentration of 1.25 mg/ml. 4 doses of 1 ml of this solution were injected intradermally at the same injection sites as the Tc-99m injections directly before surgery (total dose of 5 mg ICG). The sentinel diagnostic and extirpation procedure was started 30 minutes after the ICG injection, as the tumor excision was carried out first. We used an exoscopic ICG detection system (VITOM-ICG with D-Light P, Karl Storz GmbH, Tuttlingen, Germany). This system consists of a short exoscopic ICG telescope which is linked to a special camera and an image processing unit, presenting the ICG signals on a screen.
We incised the skin in the groin and searched for fluorescence accumulation in ICG detection mode in the groin using our standard procedure. The lymph nodes found by fluorescence were tested for radioactivity by Tc99m using a standard gamma probe (Neoprobe, Mammotome, Cincinnati, Ohio, USA) in vivo, than excised and tested again ex vivo. After removing all ICG-positive lymph nodes, we checked the groin for remaining Tc99m-positive, but ICG-negative lymph nodes and excised them, if present. At a last step, large palpable lymph nodes were excised, if found. All excised lymph nodes were sent separately to histological examination including ultrastaging. We closed the inguinal wounds and the vaginal wound using a lateral vulvar flap for reconstruction.
Results
A histologically complete tumor resection could be performed. The tumor of a clinical size of 2 cm turned out to be a bifocal carcinoma with two adjacent foci of 11 mm and 5 mm. The tumor showed DNA of HPV 16. The preoperative SPECT/CT showed an inguinal lymphatic drainage in this patient, which was expected from the tumor localization. According to this, a bilateral inguinal sentinel lymphonodectomy was performed. The sentinel lymphonodectomy using ICG could be performed without problems. In the right groin, we found two neighboring lymph nodes with ICG fluorescence, which were positive for radioactivity from Tc-99m and therefore defined as sentinel lymph nodes. No other ICG- or Tc-99m-positve lymph nodes were found in this groin (Figure 2).
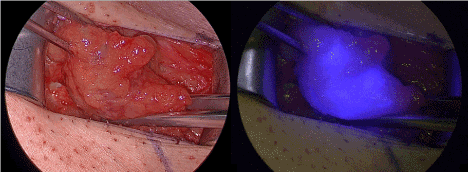
Figure 2. Right groin with sentinel lymph node under white light inspection (left) and in the near infrared fluorescence mode.
In the left groin, we located two neighboring lymph nodes with ICG fluorescence, which were positive for radioactivity from Tc-99m, too. After excision of theses lymph nodes, we found another lymph node, which showed positivity for ICG signals, but not for Tc-99m. This lymph node was excised, too, and sent to histologic examination separately.
Histologic review including ultrastaging revealed two sentinel lymph node without tumor infiltration from both sides; the ICG-positive, Tc-99m negative lymph nodes turned out to be another two lymph nodes free of tumor infiltration. Therefore, the final tumor stage was pT1b, pN0 (0/6, 0/4 SNL), G2, L0, V0, R0. We saw no adverse events or complications in this patient. The postoperative course was uneventful.
Discussion
This is the first report of a successful SLN dissection using near infrared fluorescence from ICG in vaginal cancer. All sentinel lymph nodes which were positive for Tc-99m-nanocolloide as the actual gold standard were positive for ICG, too. We found two additional ICG-positive-, but Tc-99m-negative lymph nodes in the left groin. There is only limited data about the sentinel technique in vaginal cancer. Hertel et al. [7] from our institution published 7 cases with successful sentinel detection using Tc-99m, patent blue and SPECT/CT. Pelvic sentinel lymph nodes were detected in the 6 patients with infiltration of the middle or proximal vaginal third. In one patient with the tumor site in the lower third, inguinal sentinel lymph nodes were found [7]. Frumowitz et al. [14] reported 5 cases of sentinel detection in vaginal cancer using 37 MBq of Tc-99m and could successfully detect sentinels in all cases. Van Dam et al. [15] showed a successful sentinel detection in further 4 cases using Tc-99m, too [15].
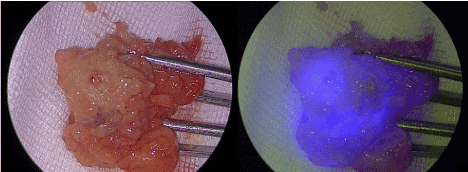
As ICG is not bound to a nanocolloid, the molecule is evacuated gradually through the lymphatic system. If the time between injection and detection is long, a complete fluorescencelymphography of the inguinofemoral lymph system will be the result. In our case, this probably led to the marking of 2 further non-sentinel lymph nodes in the left groin (as the right groin was treated before the left groin). One method to overcome this disadvantage would be to bind ICG to albumin or other nanocolloids which has been described in several smaller studies in vulvar or cervical cancer [20,21]. In vaginal cancer, a preoperative SPECT/CT or a lymphoscintigraphy is very important, as the lymphatic drainage can vary between pelvic and inguinal lymph sites, especially when the tumor localization is in the middle third of the vagina [7]. Therefore, marking with a radioactive marker should be performed with a radioactive marker in every case of vaginal cancer. But ICG has additional value in the sentinel marking. Using ICG, a visual dimension is added to the sentinel detection, so that the surgeon does not have to change between the gamma probe and surgical instruments to remove the sentinel lymph nodes. This may provide a better comfort for the surgeon. Furthermore, there is a special advantage of ICG fluorescence detection in the groin: While identifying the sentinels with the gamma probe in the groin, the probe is usually directed from laterocranial to mediocaudal which often leads to a disturbing gamma signal from the injection field at the vagina or vulva. Using ICG, this problem can be circumvented, as the ICG fluorescence shows directly the sentinel lymph nodes (Figure 3).
2021 Copyright OAT. All rights reserv
Figure 3. The same sentinel lymph node ex vivo under white light inspection (left) and in the near infrared fluorescence mode.
Our case report shows that near infrared fluorescence deriving from ICG is a promising approach for sentinel identification in vaginal cancer. If studies using sentinel lymphonodectomy will be planned, a combined approach using Tc-99m-nanocolloide and ICG could be useful.
Acknowledgements
We thank the company Karl Storz GmbH, Tuttlingen, Germany, for the granting of an ICG fluorescence diagnosis system for the realization of studies about ICG detection in gynecologic malignancies.
References
- Creasman WT, Phillips JL, Menck HR (1998) The National Cancer Data Base report on cancer of the vagina. Cancer 83: 1033-1040. [Crossref]
- Mock U, Kucera H, Fellner C, Knocke TH, Pötter R (2003) High-dose-rate (HDR) brachytherapy with or without external beam radiotherapy in the treatment of primary vaginal carcinoma: long-term results and side effects. Int J Radiat Oncol Biol Phys 56: 950-957. [Crossref]
- Chyle V, Zagars GK, Wheeler JA, Wharton JT, Delclos L (1996) Definitive radiotherapy for carcinoma of the vagina: outcome and prognostic factors. Int J Radiat Oncol Biol Phys 35: 891-905. [Crossref]
- Chang JH, Jang WI, Kim YB, Kim JH, Kim YS, et al. (2016) Definitive treatment of primary vaginal cancer with radiotherapy: multi-institutional retrospective study of the Korean Radiation Oncology Group (KROG 12-09). J Gynecol Oncol 27: e17.
- Davis KP, Stanhope CR, Garton GR, Atkinson EJ, O'Brien PC (1991) Invasive vaginal carcinoma: analysis of early stage disease. Gynecol Oncol 42: 131-136. [Crossref]
- Stock RG, Chen ASJ, Seski J (1995) A 30-year experience in the management of primary carcinoma of the vagina: analysis of prognostic factors and treatment modalities. Gynecol Oncol 56: 45-52. [Crossref]
- Hertel H, Soergel P, Muecke J, Schneider M, Papendorf F, et al. (2013) Is there a place for sentinel technique in treatment of vaginal cancer? feasibility, clinical experience, and results. Int J Gynecol Cancer 23: 1692-1698. [Crossref]
- McMahon CJ, Rofsky NM, Pedrosa I (2010) Lymphatic metastases from pelvic tumors: anatomic classification, characterization, and staging. Radiology 254: 31-46. [Crossref]
- Schnürch HG, Ackermann S, Alt CD, Barinoff J, Böing C, et al. (2016) Diagnosis, Therapy and Follow-up Care of Vulvar Cancer and its Precursors. Guideline of the DGGG and DKG (S2k-Level, AWMF Registry Number 015/059, November 2015. Geburtshilfe Frauenheilkd 76: 1035-1049. [Crossref]
- Hampl M, Hantschmann P, Michels W, Hillemanns P; German Multicenter Study Group (2008) Validation of the accuracy of the sentinel lymph node procedure in patients with vulvar cancer: results of a multicenter study in Germany. Gynecol Oncol 111: 282-288. [Crossref]
- Te Grootenhuis NC, van der Zee AG, van Doorn HC, van der Velden J, Vergote I, et al. (2016) Sentinel nodes in vulvar cancer: Long-term follow-up of the GROningen INternational Study on Sentinel nodes in Vulvar cancer (GROINSS-V) I. Gynecol Oncol 140: 8-14. [Crossref]
- Wang XJ, Fang F, Li YF (2015) Sentinel-lymph-node procedures in early stage cervical cancer: a systematic review and meta-analysis. Med Oncol 32: 385. [Crossref]
- Cibula D, Oonk MH, Abu-Rustum NR (2015) Sentinel lymph node biopsy in the management of gynecologic cancer. Curr Opin Obstet Gynecol 27: 66-72. [Crossref]
- Frumovitz M, Gayed IW, Jhingran A, Euscher ED, Coleman RL, et al. (2008) Lymphatic mapping and sentinel lymph node detection in women with vaginal cancer. Gynecol Oncol 108: 478-481. [Crossref]
- van Dam P, Sonnemans H, van Dam PJ, Verkinderen L, Dirix LY (2004) Sentinel node detection in patients with vaginal carcinoma. Gynecol Oncol 92: 89-92. [Crossref]
- Klapdor R, Hertel H, Soergel P, Hillemanns P (2016) Groin Recurrences in Node Negative Vulvar Cancer Patients After Sole Sentinel Lymph Node Dissection. Int J Gynecol Cancer 27: 166-170. [Crossref]
- Grischke EM, Röhm C, Hahn M, Helms G, Brucker S, et al. (2015) ICG Fluorescence Technique for the Detection of Sentinel Lymph Nodes in Breast Cancer: Results of a Prospective Open-label Clinical Trial. Geburtshilfe Frauenheilkd 75: 935-940. [Crossref]
- Crane LM, Themelis G, Buddingh KT, Harlaar NJ, Pleijhuis RG, et al. (2010) Multispectral real-time fluorescence imaging for intraoperative detection of the sentinel lymph node in gynecologic oncology. J Vis Exp 20: 44. [Crossref]
- Schaafsma BE, van der Vorst JR, Gaarenstroom KN, Peters AA, Verbeek FP, et al. (2012) Randomized comparison of near-infrared fluorescence lymphatic tracers for sentinel lymph node mapping of cervical cancer. Gynecol Oncol 127: 126-130. [Crossref]
- Mathéron HM, van den Berg NS, Brouwer OR, Kleinjan GH, van Driel WJ, et al. (2013) Multimodal surgical guidance towards the sentinel node in vulvar cancer. Gynecol Oncol 131: 720-725. [Crossref]
- Hutteman M, van der Vorst JR, Gaarenstroom KN, Peters AA, Mieog JS, et al. (2012) Optimization of near-infrared fluorescent sentinel lymph node mapping for vulvar cancer. Am J Obstet Gynecol 206: 89. [Crossref]



 2021 Copyright OAT. All rights reserv
2021 Copyright OAT. All rights reserv