Background: Behcet's Disease (BD) is a chronic, relapsing, systemic vasculitis of unknown etiology. Patients with BD have increased insulin resistance (IR) and susceptibility to the Metabolic Syndrome (MetS). The association of the hepcidin molecule, which is known to be an antimicrobial peptide, with IR has been recently reported. This study aims to determine the serum hepcidin levels of patients with BD and healthy controls, and investigate the association of IR and MetS.
Methods: Twenty patients with BD and 20 healthy individuals as the control group were enrolled in this study. Serum hepcidin levels of all subjects were measured simultaneously with the ELISA method.
Results: No statistically significant difference was found between patients with BD and the controls in terms of hepcidin levels (p>0.05). No statistically significant difference was found between patients with BD presenting with and without MetS with respect to the mean serum hepcidin level (p>0.05). A combined evaluation of patients with BD and the controls with IR and/or MetS. Serum hepcidin (p=0.004) level and the hepcidin/ferritin ratio (p=0.001) were significantly low, and the levels of serum ferritin (p=0.008) was significantly high in the group with IR and/or MetS.
Conclusion: We found that the hepcidin level of patients with BD was not different compared with the controls, there was no significant difference between patients with BD presenting with IR and/or with MetS in terms of serum hepcidin levels, and serum ferritin levels of patients with BD increased and hepcidin/ferritin ratios significantly decreased compared with the controls.
Behcet’s disease, hepcidin, metabolic syndrome, hepcidin, ferritin ratio
Behcet's disease (BD) is a chronic, relapsing, systemic vasculitis of unknown etiology with the clinical features of mucocutaneous lesions, ocular, vascular, articular, gastrointestinal, urogenital, pulmonary, and neurologic involvement [1]. There are recent publications showing increased insulin resistance (IR) in patients with BD. These publications have suggested that chronic inflammation caused by increased cytokines (especially IL-6) in BD resulted in increased IR and metabolic syndrome (MetS) disorder [2,3].
Metabolic syndrome is an important public health problem characterized by hyperglycemia, dyslipidemia, hypertension, and obesity, also causing cardiovascular risk and nonalcoholic fatty liver disease. Overweight patients with abnormal glucose metabolisms and dyslipidemia are histologically known to have iron overload in their livers [4]. This condition has been described as dysmetabolic iron overload syndrome (DIOS), or insulin resistance-associated hepatic iron overload (IRHIO). The pathophysiological link between hepatic iron overload and MetS has not yet been clearly defined, however studies reporting that there is a complex interaction between insulin/IGF-1 signaling and ferritin expression tended to investigate the hepcidin molecule [5].
Hepcidin has initially been identified as an antimicrobial peptide in human serum and urine. Then, it has been found to act as a hormone regulating iron homeostasis in mammals [6,7]. Bacteria are known to reproduce more rapidly in iron-rich media. Levels of hepcidin are elevated during infection and inflammation, resulting in the stimulation of inflammatory cytokines. This helps them the iron to be kept by the cells and prevents microorganisms from growing by inhibiting iron flow to the plasma. So, a decrease is observed in the iron level in case of inflammation, chronic diseases and obesity [8,9].
In this study, we aimed to determine the serum hepcidin level in patients with BD and planned to investigate the relationship of the hepcidin molecule between patients with BD presenting with and without MetS and healthy controls.
Selection of cases and their characteristics
This study was conducted in a total of 20 patients with BD, (11 male and 9 female), diagnosed according to the International Study Group Criteria for Behçet’s Disease [10] and 20 healthy individuals who were followed up by the Dermatology Clinic, Faculty of Medicine, Firat University. The local ethical committee approved this study under decision no 0500104-112 of 09.06.2011, and the data were collected from the archives. The written consents were obtained from all participants. Age and sex of patients, disease duration and the treatments received by the patients were recorded. Symptoms and/or findings of BD (oral aphthae, genital ulcers, eye involvements, arthralgia, arthritis, acneiform skin lesions, lesions similar to erythema nodosum, thrombophlebitis, gastrointestinal involvements, neurological involvements and pathergy test results) were recorded. And the healthy control group was composed of 20 healthy individuals, (7 male and 13 female) who had no systemic, dermatologic, rheumatologic and neurologic diseases (including family history), who were non-alcohol users, who did not take narcotic drugs and medications, who did not present with anemia, vitamin 12 and folic acid deficiency, and who admitted to our hospital for annual check-ups.
Fasting blood glucose, triglyceride, LDL, VLDL, HDL and lipid profile including the total cholesterol, HbA1c, insulin, C-peptide levels of patient and control groups were measured and Body Mass Index (BMI), waist circumference, blood pressure measurements were done.
Body Mass Index was calculated using the formula weight/height squared [11]. The participants were divided into five categories: 'underweight' (BMI <20 kg/m2) (1), 'normal' (BMI 20-25 kg/m2) (2), 'overweight' (BMI 25-30 kg/m2) (3), 'obese' (BMI 30-40 kg/m2) (4), and 'morbidly obese' (BMI >40 kg/m2) (5). MetS Diagnostic Criteria according to the definition of the International Diabetes Foundation (IDF)-2005 were used for the diagnosis of MetS [12].
Waist circumference measurements of patient and control groups were performed in standing position with a soft measuring tape on the middle line between the lowest costa and crista iliaca [13]. Homeostasis model assessment of insulin resistance formula (HOMA-IR) (0.sec glucose mg/dlx0.sec insulin U/ml)/405 was used to calculate IR. Cases with HOMA-IR>3.2 were diagnosed with IR [14].
Laboratory assessment
Because hepcidin is a peptide hormone and can be broken down by proteases, aprotinin (500 Kallikrein units per ml) was added to the tubes before blood sample collection to prevent proteolysis [15].
Five mL blood samples were collected from each subject on empty stomach, placed into Eppendorf tubes after centrifugation, and stored at -80°C in deep freezer. Serum hepcidin (human hepsidin-25cat no: CK-E90211; Eastbiopharm, China) levels were measured with the ELISA method and as stated in the manufacturer’s manual.
Statistical analysis
SPSS version 12.0 was used for statistical analyses. Results obtained in this study were expressed as mean ± SD, and Mann-Whitney U test was performed for intra-group comparisons. P values less than 0.05 levels were considered to be statistically significant.
Twenty patients with BD (11 males and 9 females) and 20 healthy individuals for control group (9 males and 11 females) were enrolled in this study. There was no statistically significant difference between the groups with respect to age and gender distribution (p>0.05). The mean age of the participants was 35.10 ± 6.8 years for the patients with BD, and 31.90 ± 7.0 years in the control group. There was no significant difference among the groups in terms of mean age (p>0.05). Similarly, there were no significant differences in BMI and waist circumference between the BD (24.29 ± 1.30 kg/m2, 86.05 ± 6.50), and control groups (24.06 ± 2.47 kg/m2, 82.20 ± 11.95) (p>0.05). Disease duration was 5.80 ± 4.39 years in the patients with BD.
Symptoms and/or findings belonging to the patients with BD were recorded as active, inactive (undergone) or never observed. All patients with BD had oral aphthae, 13 (65%) were active, 7 (35%) were inactive; genital ulcer was active in 7 (35%) patients, inactive in 13 (65%) patients, eye involvement was active in 2 (10%) patients, inactive in 7 (35%) patients. Joint involvement was active in 6 patients (30%), inactive in 5 patients (25%), erythema nodosum was active in 4 (20%) patients, inactive in 4 patients (20%), papulopustular lesions were active in 5 (25%) patients, inactive in 5 (25%) patients, superficial thrombophlebitis was active in 4 (20%) patients, inactive in 2 (10%) patients. Moreover, 4 patients had cardiovascular, 1 patient had gastrointestinal system involvement, 3 patients had central nervous system involvement and 15 (75%) patients had positive pathergy.
No statistically significant difference was found between the patients with BD and the controls in terms of mean hepcidin levels (p<0.05). In patients with BD serum iron binding capacity and hepcidin/ferritin ratio was found to be significantly low compared with the controls and serum ferritin was found high (respectively p=0.001, p=0.01 and p=0.008) (Table 1, Figure 1). When laboratory values were examined with respect to the gender, no statistically significant difference was found (p<0.05).
Figure 1. Hepcidin/ferritin ratio in patients with BD and the control group
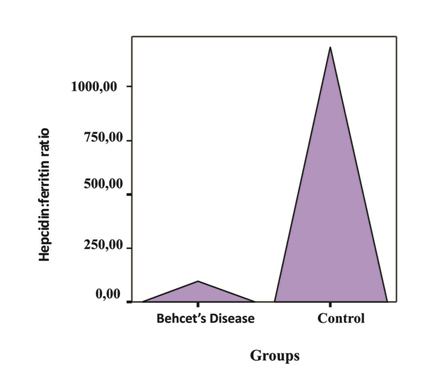
Table 1. Laboratory values of patients with BD and the control group
|
Behcet’s Disease (n=20) |
Control (n=20) |
p |
Serum hepcidin (ng/mL) |
1003.81 ± 1073.65 |
1313.71 ± 1111.43 |
p>0.05 |
Ferritin* (ng/mL) |
95.80 ± 90.92 |
33.35 ± 45.72 |
p=0.008 |
Hepsidin:ferritin ratio* |
93.63 ± 330.23 |
1182.89 ± 2562.28 |
p=0.01 |
IBC* (µg/dL) |
275.20 ± 94.55 |
373.05 ± 56.17 |
p=0.001 |
Glucose* (mg/dL) |
82.85 ± 12.03 |
83.25 ± 10.83 |
p>0.05 |
Triglycerides* (mg/dL) |
134.40 ± 57.09 |
103.00 ± 40.57 |
p>0.05 |
LDL-cholesterol* (mg/dL) |
114.14 ± 39.69 |
115.30 ± 34.56 |
p>0.05 |
HDL-cholesterol* (mg/dL) |
42.62 ± 9.84 |
48.10 ± 12.06 |
p>0.05 |
Total cholesterol* (mg/dL) |
179.35 ± 42.28 |
167.05 ± 34.05 |
p>0.05 |
Insulin* (µIU/mL) |
8.00 ± 7.59 |
6.87 ± 3.67 |
p>0.05 |
C-peptid* (ng/mL) |
2.37 ± 1.30 |
1.65 ± 0.45 |
p>0.05 |
HbA1C |
4.73 ± 0.22 |
4.70 ± 0.31 |
p>0.05 |
HOMA-IR values* |
1.67 ± 1.58 |
1.40 ± 0.73 |
p>0.05 |
*Mean ± SD, IBC: Iron Binding Capacity, HbA1C: Hemoglobin A1C, HOMA-IR: Homeostasis
Model Assessment of Insulin Resistance.
Eight of the patients with BD (40%), 6 of the controls (30%) presented with MetS. No statistically significant difference was found between the patients with BD presenting with and without MetS in laboratory parameters (p<0.05). The mean age of patients with BD presenting with MetS was 39.87 ± 6.4, with a disease duration of 8.87 ± 4.76, and these were found to be significantly high (31.96 ± 5.1, 3.75 ± 2.73) compared with subjects without MetS (p= 0.007, p=0.02) (Table 2).
Table 2.The distribution of laboratory values according to the presence of MetS and IR in patients with BD
Behcet’s Disease |
Hepcidin* (ng/mL) |
Ferritin* (ng/mL) |
Hepsidin:ferritin ratio* |
p |
MetS |
Positive (n=8) |
991.82 ± 988.15 |
108.96 ± 109.99 |
17.59 ± 15.77 |
p>0.05 |
Negative (n=12) |
1011.81 ± 1170.27 |
87.03 ± 79.82 |
144.33 ± 425.67 |
p>0.05 |
IR |
Positive (n=5) |
597.26 ± 109.90 |
86.38 ± 70.59 |
12.19 ± 9.60 |
p>0.05 |
Negative (n=15) |
1139.35 ± 1217.48 |
98.94 ± 98.76 |
120.78 ± 380.55 |
p>0.05 |
MetS and/or IR |
Positive (n=9) |
862.73 ± 855.88 |
112.58 ± 97.64 |
14.30 ± 14.43 |
p>0.05 |
Negative (n=11) |
1176.24 ± 1327.12 |
75.30 ± 82.80 |
190.59 ± 489.40 |
p>0.05 |
*Mean ± SD, MetS: Metabolic Syndrome, IR: Insulin Resistance
Five of the patients with BD (25%) were insulin resistant. No significant difference was found especially in terms of serum hepcidin, the hepcidin/ferritin ratio and other laboratory parameters between the patients with BD with and without IR (Table 2).
There was a total of 11 (55%) in the group of patients with BD presenting with IR and/or MetS. The disease durations of these patients were 7.81 ± 4.5 on average and were significantly higher (3.33 ± 2.6) than the ones without (p=0.02), no significant difference was found in terms of other laboratory parameters (especially serum hepcidin and hepcidin/ferritin ratio) (Table 2).
A combined evaluation of patients with BD and the controls found 14 (%35) MetS, 6 (%15) IR, and 18 (45%) subjects with IR and/or MetS. Serum hepcidin (p=0.004) level and the hepcidin/ferritin ratio (p=0.001) were significantly low, and the levels of serum ferritin (p=0.008) was significantly high in the group with IR and/or MetS (Table 3).
Table 3. The distribution of laboratory values according to the presence of MetS and IR in patients with BD and the control group
Behcet’s Disease and
Control Group |
Hepcidin* (ng/mL) |
Ferritin* (ng/mL) |
Hepsidin:ferritinratio* |
p |
MetS |
Positive (n=14) |
805.99 ± 762.09a |
91.66 ± 93.79 |
25.93 ± 37.76b |
ap=0.02
bp=0.02 |
Negative (n=26) |
1348.71 ± 1201.69a |
49.99 ± 65.01 |
967.98 ± 2285.45b |
IR |
Positive (n=6) |
764.35 ± 420.93
|
77.13 ± 67.08 |
18.79 ± 18.29 |
|
Negative (n=34) |
1228.36 ± 1159.26 |
62.36 ± 80.19 |
747.58 ± 2209.82 |
|
MetS and/or IR |
2021 Copyright OAT. All rights reserv
Positive (n=18) |
802.18 ± 704.17c |
93.38 ± 87.80d |
23.97 ± 34.64e |
cp=0.003
dp=0.008
ep=0.001 |
Negative (n=22) |
1450.51 ± 1267.57c |
41.01 ± 60.78d |
1140.87 ± 2452.47e |
*Mean ± SD, MetS: Metabolic Syndrome, IR: Insulin Resistance.
Serum ferritin level was negatively correlated with iron binding capacity and serum folic acid, and was positively correlated with the mean age values of patients and the duration of disease (r=0.04 at p<0.05, r=0.002 at p<0.05, r=0.002 at p<0.001, respectively).
We particularly investigated the change in serum hepcidin levels between the patients with BD presenting with and without MetS. No difference was found between patients with BD presenting with and without MetS in addition to no differences in serum hepcidin levels between the patients with BD and the controls. The mean age, thus the duration of disease, in patients with BD presenting with MetS were found to be significantly high similar to that of the general population only. A similar condition was found in patients with BD with or without insulin resistance, and at-risk and no-risk patients for waist circumference. However, a combined evaluation of patients with BD and the controls revealed the blood hepcidin level to be significantly low in subjects at-risk (waist circumference: ≥ 94 cm in European men, ≥ 80 cm in women) compared with subjects with no-risk for waist circumference. Similarly, a combined evaluation of patients with BD and the controls found that serum hepcidin level was significantly low in the group with IR and/or MetS.
One of the manifestations of MetS, obesity is associated with chronic inflammation and iron deficiency. Adipose tissue hypoxia is considered to lead to the chronic inflammation observed in obesity. Adipose tissue is a very active endocrine organ, secreting numerous hormones and cytokines associated with important systemic effects on different metabolic processes [16,17]. The literature contains various studies investigating the relationship between obesity, inflammation, and iron deficiency. The prevalence of iron deficiency without anemia (IDNA) is highest in this period when the body fat is fairly high in preadolescents [18]. Tussing-Humphreys et al. [19] have reported raised levels of CRP, IL-6 and hepcidin, an impaired iron absorption and a positive correlation between hepcidin level and IL-6 level in morbid obese women compared with Hb-matched, non-obese controls. CRP and IL-6 have also been reported to significantly decrease with weight loss in the obese who have undergone bariatric surgery [20].
Martinelli et al. [21] have reported that serum hepcidin and ferritin levels in a population of 1,391 subjects were significantly higher in subjects with MetS compared with subjects without and that serum hepcidin level was increased with findings of MetS. This study emphasizes that the pleiotropic effect of hepcidin would exacerbate IR and could contribute to the appearance of cardiovascular complications.
A literature review an increase in hepcidin level in patients with MetS was reported in many studies. However, in polycystic over syndrome (PCOS), which is another disease in association with IR and abnormal glucose tolerance, serum ferritin levels were reported to increase, hepcidin level to decrease and a mild iron overload was noted. It has been stated that iron caused an increase in intestinal absorption and iron overload could to lead to IR and diabetes mellitus depending on decreased negative effects of the decrease in hepcidin level on ferroportin [22]. In a similar study, no significant difference was found between patients with PCOS and the controls with respect to serum hepcidin levels [23]. Fernandez et al. [24] have reported that iron and glucose metabolisms were correlated, iron affected insulin secretion and sensitivity, and insulin affected the iron metabolism. In this study, similar to the study of Gozdemir et al., [23] no significant difference was observed between the patients with BD presenting with IR and/or MetS in terms of serum hepcidin levels.
Tan et al. [25] have reported that serum hepcidin levels decreased in chronic liver patients, another inflammatory disease, but this decrease was not significantly different compared with the controls. Interestingly in this study, the hepcidin/ferritin level was significantly low in chronic liver patients, and the hepcidin/ferritin ratio was found to be related with the increase in the fibrosis stage. In this study, hepcidin/ferritin ratio was reported to be an important evidence for progressive fibrosis in chronic liver diseases. This study also noted that serum ferritin increased and hepcidin/ferritin ratio decreased significantly in patients with BD compared with the controls. We consider this to be due tothe increase in ferritin, an acute phase reactant in patients with BD. Interestingly in this study, a combined evaluation of patients with BD and the controls showed that serum hepcidin level and hepcidin/ferritin ratiosdecreased significantly in the group with IR and/or MetS.
In conclusion, the hepcidin molecule, which has been known as an antimicrobial peptide for years, has been recently identified to have an association with IR. There are recent publications showing increased IR in patients with BD. We found that serum hepcidin level was not different in patients with BD compared with the controls, and that there was no significant difference in serum hepcidin levels between the patients with BD presenting with and without IR and/or MetS. We conclude that serum ferritin level increased in patients with BD and hepcidin/ferritin ratios significantly decreased compared with the controls.
This study was supported financially by the Firat University Research Project Unit (project number: TF.11.81).
- Pineton de Chambrun M, Wechsler B, Geri G, Cacoub P, Saadoun D (2012) New insights into the pathogenesis of Behçet's disease. Autoimmun Rev 11: 687-698. [Crossref]
- Oguz A, Dogan EG, Uzunlulu M, Oguz FM (2007) Insulin resistance and adiponectin levels in Behçet's syndrome. Clin Exp Rheumatol 25: S118-119. [Crossref]
- Erdem H, Dinc A, Pay S, Simsek I, Turan M (2006) Peripheral insulin resistance in patients with Behçet's disease. J Eur Acad Dermatol Venereol 20: 391-395. [Crossref]
- Moirand R, Mortaji AM, Loréal O, Paillard F, Brissot P, et al. (1997) A new syndrome of liver iron overload with normal transferrin saturation. Lancet 349: 95-97. [Crossref]
- Dongiovanni P, Fracanzani AL, Fargion S, Valenti L (2011) Iron in fatty liver and in the metabolic syndrome: a promising therapeutic target. J Hepatol 55: 920-932. [Crossref]
- Kemna EH, Tjalsma H, Willems HL, Swinkels DW (2008) Hepcidin: from discovery to differential diagnosis. Haematologica 93: 90-97. [Crossref]
- Park CH, Valore EV, Waring AJ, Ganz T (2001) Hepcidin, a urinary antimicrobial peptide synthesized in the liver. J Biol Chem 276: 7806-7810. [Crossref]
- Viatte L, Vaulont S (2009) Hepcidin, the iron watcher. Biochimie 91: 1223-1228. [Crossref]
- Nemeth E, Valore EV, Territo M, Schiller G, Lichtenstein A, et al. (2003) Hepcidin, a putative mediator of anemia of inflammation, is a type II acute-phase protein. Blood 101: 2461-2463. [Crossref]
- [No authors listed] (1990) Criteria for diagnosis of Behçet's disease. International Study Group for Behçet's Disease. Lancet 335: 1078-1080. [Crossref]
- Sterry W, Strober BE, Menter A; International Psoriasis Council (2007) Obesity in psoriasis: the metabolic, clinical and therapeutic implications. Report of an interdisciplinary conference and review. Br J Dermatol 157: 649-655. [Crossref]
- Lawlor DA, Smith GD, Ebrahim S (2006) Does the new International Diabetes Federation definition of the metabolic syndrome predict CHD any more strongly than older definitions? Findings from the British Women's Heart and Health Study. Diabetologia 49: 41-48. [Crossref]
- Celik O, Celik N, Hascalik S, Sahin I, Aydin S, et al. (2011) An appraisal of serum preptin levels in PCOS. Fertil Steril 95: 314-316. [Crossref]
- Kondo N, Nomura M, Nakaya Y, Ito S, Ohguro T (2005) Association of inflammatory marker and highly sensitive C-reactive protein with aerobic exercise capacity, maximum oxygen uptake and insulin resistance in healthy middle-aged volunteers. Circ J 69: 452-457. [Crossref]
- Stengel A, Keire D, Goebel M, Evilevitch L, Wiggins B, et al. (2009) The RAPID method for blood processing yields new insight in plasma concentrations and molecular forms of circulating gut peptides. Endocrinology 150: 5113-5118. [Crossref]
- Dandona P, Aljada A, Bandyopadhyay A (2004) Inflammation: the link between insulin resistance, obesity and diabetes. Trends Immunol 25: 4-7. [Crossref]
- Gaffney-Stomberg E, McClung JP (2012) Inflammation and diminished iron status: mechanisms and functional outcomes. Curr Opin Clin Nutr Metab Care 15: 605-613. [Crossref]
- Sanad M, Osman M, Gharib A (2011) Obesity modulate serum hepcidin and treatment outcome of iron deficiency anemia in children: a case control study. Ital J Pediatr 37: 34. [Crossref]
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Guzman G, et al. (2010) Elevated systemic hepcidin and iron depletion in obese premenopausal females. Obesity (Silver Spring) 18: 1449-1456. [Crossref]
- Tussing-Humphreys LM, Nemeth E, Fantuzzi G, Freels S, Holterman AX, et al. (2010) Decreased serum hepcidin and improved functional iron status 6 months after restrictive bariatric surgery. Obesity (Silver Spring) 18: 2010-2016. [Crossref]
- Martinelli N, Traglia M, Campostrini N, Biino G, Corbella M, et al. (2012) Increased serum hepcidinlevels in subjects with the metabolic syndrome: a population study. PLoSOne 7: e48250. [Crossref]
- Escobar-Morreale HF (2012) Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol Metab 23: 509-515. [Crossref]
- GÃzdemir E, Kaygusuz I, KafalÄ H (2013) Is hepcidin a new cardiovascular risk marker in polycystic ovary syndrome? Gynecol Obstet Invest 75: 196-202. [Crossref]
- Fernández-Real JM, López-Bermejo A, Ricart W (2002) Cross-talk between iron metabolism and diabetes. Diabetes 51: 2348-2354. [Crossref]
- Tan TC, Crawford DH, Franklin ME, Jaskowski LA, Macdonald GA, et al. (2012) The serum hepcidin:ferritin ratio is a potential biomarker for cirrhosis. Liver Int 32: 1391-1399. [Crossref]

