Purpose: In order to further elucidate the efficacy and safety of some nutritional supplements on gonarthrosis, we have conducted a preliminary randomized multicenter (n=9) observational study comparing the effects of an association of Fortigel® (10 gr) and Fucoidan (100 mg) (ACTEN®) versus another commonly therapeutically used formulation based on Glucosamine (500 mg), Chondroitin Sulfate (400mg) hyaluronic Acid (50 mg) and Vitamin C (100 mg) (COMBIART).
Patients and methods: The protocol was administered over a 12-weeks period in a population (n=126) aged 40-65 years, with diagnosed mild-to-moderate osteoarthritis (OA) of the knee (grade 2-3 of Kellgren Lawrence grading scale). Safety was measured by closely monitoring adverse events. Efficacy was measured by grading evaluations, at basal, 1 month and 3 months controls, of the Visual Analog Scale (VAS) and the Lequesne algofunctional index for severity of osteoarthritis (LAI) for articular functionality.
Results: Both groups showed an important reduction (P < 0.0001) in the mean visual analog scale values at T1 (28.5% ACTEN®, 21.3% COMBIART at 1 month) and T3 (49.4% ACTEN®, 40.1% COMBIART at 3 months), as well as a marked reduction in the Lequesne algofunctional index means (P < 0.0001) (ACTEN® 28.9% T1 44.9% T3, COMBIART T1 21.3% T3 37%). The effect seems to be time dependent, as the mean values decrease further for both parameters from T1 to T2 (P < 0.0001, for VAS for both groups; P 0.0011 for ACTEN® group, P 0.0064 Control group for LAI). No statistically significant difference was found between the ACTEN® group and the COMBIART group at time T1 or T3. These interesting preliminary data will be further investigated on a larger scale.
Conclusions: Fortigel® (10gr) and Fucoidan (100 mg) (ACTEN®) taken as oral nutritional supplements have a significant impact as therapeutic intervention for knee osteoarthritis as indicated by the marked decrease in VAS and LAI values over the course of the treatment. A similar effect, as expected, has been confirmed in the COMBIART group, and no statistically significant difference has been detected between the two groups.
Osteoarthritis (OA) is a very common, slowly progressive joint condition, that causes significant discomfort and disability in the adult population. The prevalence of the condition increases with age, with well-known risk factors such as genetics, obesity, local trauma, and occupation. Important radiographic changes of knee OA are found in 1% of adults of 25–35 years of age and increase almost to 50% in elders aged 75 years and older. OA of the hips and knees are the most common complaint in the adult population.
Osteoarthritis has an inflammatory component, and a tissue breakdown component, which result in pain and stiffness [1] in these crucial weight-bearing joints. This often imposes an important disability, leading to an increased difficulty in undertaking normal activities of daily life, up to requiring joint replacement surgery in the most severe of cases.
Acetaminophen and nonsteroidal anti-inflammatory drugs (NSAIDs) are common treatments for patients with osteoarthritis to cope with pain and stiffness, in severe conditions on a daily basis. For this reason, in recent years many studies have focused on alternative molecules and nutritional supplements as therapeutic options to achieve cartilage tissue repair, while bypassing the negative effects of cyclooxygenase 2 (COX-2) inhibitors and other NSAIDs. Glucosamine has been extensively studied [2-18] showing positive results in the clinical management of pain and structural improvements [19]. Chondroitin sulfate coupled to Glucosamine, or by itself, has been the center of significant studies [20], but many other compounds synthetic or natural have been investigated, including Vitamin C [21-25]. Particular interest has been paid to Collagen hydrolysates (CHs) and Fucoidans [26-44]. CH is obtained by the enzymatic hydrolysis of collagenous tissues from mammals. The main characteristic of CH is its amino acid composition, which is identical to type II collagen, thus providing high levels of glycine and proline, essential amino acids for the stability and regeneration of cartilage [28]. This product is recognized as a safe food ingredient by regulatory agencies. CH is well digested and is preferentially accumulated in cartilage [29]. Clinical use of CH has not been associated with adverse effects, aside some gastro-intestinal side effects, such as fullness or unpleasant taste.
Fucoidans, on the other side, are a class of sulfated, fucose-rich polymers found in several types of brown macroalgae [41,42] used in a variety of different medical conditions [38]. Several recent studies indicate a role of fucoidan in addressing the symptoms of osteoarthritis. Animal models of collagen induced arthritis showed that orally administered fucoidans successfully inhibited pain [39]. In a small human clinical study osteoarthritis symptoms were inhibited by 12 weeks oral administration of fucoidan rich seaweed extracts by 52% [40]. There was no reduction in TNF alpha as inflammation marker, but an accompanying study in healthy volunteers showed a decrease in Interleukin 6, a marker for chronic inflammation [43]. Fucoidan's effect on pain has been linked to its selective blockade on neutrophils accumulation [44,45].
In order to test the clinical efficacy of the combined use of these compounds, CH (Fortigel® 10gr.) and Fucoidan (100mg) (ACTEN®), we have designed a preliminary multicenter randomized clinical trial. Testing its clinical efficacy against another formulation used in clinical practice, based on well-known substances: Glucosamine (500mg), Chondroitin Sulfate (400mg) hyaluronic Acid (50 mg) and Vitamin C (100mg) (COMBIART).
This trial was designed as a randomized preliminary study. It was conducted over 12 weeks (84 days) by a single research group in nine centers (Lazio and Sardinia regions, in Italy) between Oct 2015 and May 2016 and involved 126 participants aged 40-65 years, with mild-to-moderate osteoarthritis (OA) of the knee (grade 2-3 of Kellgren Lawrence grading scale).
Individuals were excluded from the study if they 1) were aged 39 years or younger at the start of the study; 2) had rheumatoid or other forms of arthritis; 3) had joint pain as a result of nerve or muscle damage, accidents, falls, trauma, etc. (4) had comorbidities often associated with osteoarthritis, such as diabetes, cardiovascular disease, elevated cholesterol requiring medical intervention, renal insufficiencies, asthma, or hypertension requiring medical intervention; 5) had cancer within the prior 5 years; 6) were taking any other herbal product or other supplement for pain or inflammation of joints health, such as glucosamine/chondroitin/methylsulfonylmethane, S-adenosylmethionine, or omega-3; 7) were smokers; 8) were heavy alcohol consumers; 9) were pregnant or nursing women; 10) had shellfish allergies; and 11) had a BMI lower than 18.5 (underweight) or greater than 40.0 (morbidly obese) or a body weight exceeding 225 lbs (102 kg). The demographics of the patient population are listed in Table 1.
Table 1. The demographics of the patient population.
Population |
|
|
Age |
Mean 57.10 |
SD 8.04 |
Men |
48 |
|
Women |
78 |
|
VAS cm |
Mean 6.595 |
SD 1.318 |
LAI (Lequesne index) |
Mean 10.595 |
SD 3.519 |
Intervention
Participants underwent a 4-week washout period during which they stopped taking all dietary supplements for bones, joints, and inflammation as well as non-prescription drugs.
Prescription drugs that were not related to joint health were allowed, as was OTC rescue medication that was taken only on an as-needed basis. Participating individuals were also asked not to change any aspects of their lives during the 4-week trial. Lifestyle variables were unaltered, including but not limited to diet, fitness regimens, work and family-related tasks.
Participants who passed the initial screening at the baseline appointment were provided with one of the two randomly assigned nutritional supplements (ACTEN® or COMBIART), paper copies of a visual analog scale (VAS) questionnaire and the Lequesne algo-functional index (LAI). Each participant was asked to fill out the baseline VAS and LAI questionnaire and return it to the investigator before beginning to take the supplements. The supplements were consumed daily for both groups for the first month. The remaining eight weeks ACTEN® group used the supplement every other day, while COMBIART continued on a daily basis.
Data were collected again at 1 month and 3 months (12 weeks). This approach was chosen to reflect the higher absorption of gel (ACTEN®) compared to capsules (COMBIART) [46].
Outcome measures
A VAS scale of 0 to 10 for pain, and the LAI scale of 0 to 24 for pain and functionality were employed to evaluate the 2 measures before and after 4 and 12 weeks of supplementation. VAS scores were reported by asking the participants to mark responses on a 10-cm line. The line contained both end anchor points and 3 additional descriptors that were evenly spaced along the VAS scale at 2.5-cm intervals to help orient the participant. Definitions for the descriptors for each scale are listed in Table 2.
Table 2. Internal descriptor for the VAS Scale, descriptors for LAI are as previously published [45].
VAS Value (cm) |
Pain Level |
0 |
None |
2.5 |
Mild |
5 |
Moderate |
7.5 |
Severe |
10 |
Worst possible |
At baseline evaluation, participants were told to notify the research team immediately if any mild, moderate, or severe adverse events occurred during the period of supplementation. During the 1 month follow-up, and again at 3 months participants were once again asked whether they had experienced any adverse events or required any form of rescue treatment (pain or anti-inflammatory drugs) during the supplementation period. No complaints were reported.
The criteria for mild, moderate, and severe adverse events were as follows: (1) mild: an adverse event that does not interfere with usual day-to-day activities and requires no special intervention or treatment; (2) moderate: an adverse event that can affect usual daily activities and that can be addressed with simple therapeutic treatments; (3) severe: an adverse event that requires therapeutic intervention.
Statistical analysis
Data gathered from the VAS and LAI questionnaires underwent statistical analysis using a 2-tailed, paired t test to test baseline versus T1 (1 month) and T2 (3 months) for both treatments. Data were cross-analyzed using a 2-tailed non-paired t test to compare efficacies. The analyses were performed with the Graphpad PRISM statistical analysis software, version 5.0 (La Jolla, CA, USA). The alpha that was used for statistical significance was 0.05.
Of the 126 participants enrolled in the study, none withdrew or were removed due to medical complications or lack of compliance with the study’s protocol. In comparison with baseline, the results for all participants at the end of the 4/12 weeks for all measures were statistically significant. The ACTEN® group showed a 28.5% decrease in joint pain at 1 month (T1), from a mean VAS score of 6.68 (1.38SD) at baseline to 4.77 (1.98SD) (P = 0.0001; 95% CI, 1.57, 2.25) and a -49.4% at T3, mean VAS 3.38 (1.78SD) (P = 0.0001; 95% CI, 2.95, 3.65). LAI was also reduced by -28.9% at T1, from a mean baseline of 11.28 (3.69SD) to 8.01 (P = 0.0001; 95% CI, 2.72, 3.82) and by -44.9% at T3 mean LAI 6.21 (3.01SD) (P = 0.0001; 95% CI, 4.42, 5.71).
The COMBIART group showed a 21.3% decrease in joint pain, from a mean VAS score of 6.5 (1.25SD) at baseline to 5.11 (1.53SD) at T1 (P = 0.0001; 95% CI, 1.09, 1.69) and a – 40.1% at T3 mean VAS 3.89 (1.81SD) (P = 0.0001; 95% CI, 2.97, 4.33). LAI is again reduced by -21.3% at T1 from a mean baseline of 9.84 (3.18 SD) to 7.74 (3.08 SD) (P = 0.0001; 95% CI, 1.63, 2.57) and by -37% at T3, mean LAI 6.19 (3.04 SD) (P = 0.0001; 95% CI, 2.97, 4.33)
The ACTEN® group seems to work more efficently than the COMBIART group (VAS T3 means -49.4%/-40.1%; LAI T3 means -44.9%/-37%), but no statistically relevant significance has been detected comparing the two groups [Figure 1-4].
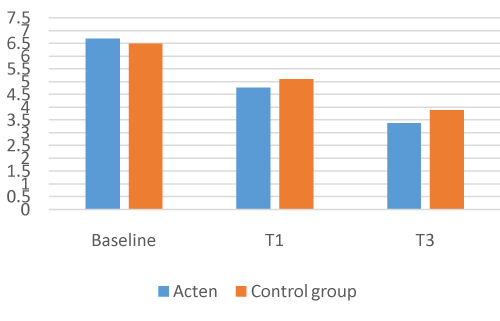
Figure 1. VAS Acten vs Control Group.
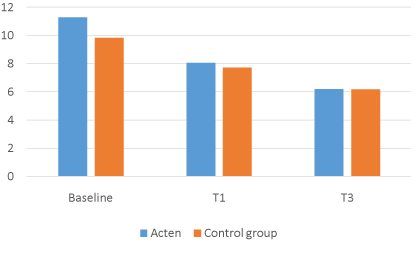
Figure 2. Lequesme Index Acten vs COMBIART.
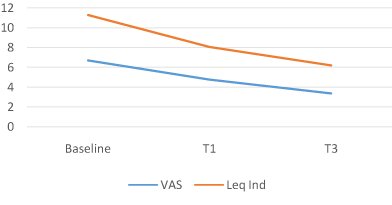
Figure 3. ACTEN Group.
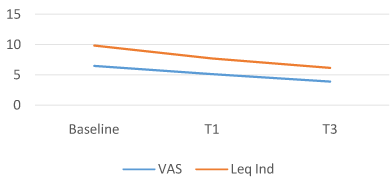
Figure 4. COMBIART Group.
Summary of adverse effects
No adverse effects were reported during the study.
2021 Copyright OAT. All rights reserv
Osteoarthritis is the most common form of arthritis in Italy and in the USA [47]. Glucosamine and chondroitin sulfate and hyaluronic acid are natural substances present in and around cartilage cells and, as such, are the most commonly used natural supplements for OA and joint pain. Previous studies have found them to be effective and safe for addressing the symptoms of OA [48,49]. In particular, the Glucosamine/Chondroitin Arthritis Intervention Trial in 2006, sponsored by the National Institutes of Health (NIH), found that glucosamine combined with chondroitin sulfate provided statistically significant pain relief for a subset of participants in comparison with a placebo [20].
In this study we have evaluated the clinical safety and efficacy of ACTEN® (CH 10gr and Fucose 100 mg) and compared it with a supplement (COMBIART) based on the well- studied effects: glucosamine, chondroitin sulfate, hyaluronic acid and the added value of Vitamin C [21]. As expected the COMBIART group showed a marked reduction in pain and functionality indexes. The decrease in pain and functionality is established in the first 4 weeks of treatment (-21.3% for both VAS and LAI) and is further increased in the following weeks, up to a -40.1% in VAS and -37% in LAI at T3 (12 weeks). ACTEN® showed similar results, with a reduction in VAS and LAI at T1 of 28.5% and 28.9% respectively. Again the highest efficacy is reached with prolonged exposure, reaching a -49.4% in VAS and -44.9% in LAI at T3. Notably the ACTEN® group in the last 8 weeks only assumed the nutritional supplement once every other day, without any loss of efficacy. No statistically relevant difference has been found between the two groups, but larger numbers or prolonged exposures to the supplements might uncover subtler differences.
It has to be taken in consideration that, a daily regimen of ACTEN®, instead of every other day regimen as used in this trial, it is likely to produce further benefits, and it should be investigated.
Both nutritional supplements (ACTEN® and COMBIART) are safe, well tolerated, and able to exert an important pain reduction and improved functionality in the OA of the knee. Further studies are required to establish pathways for the shown efficacy in pain reduction and radiological studies to check on the structural changes behind the improved functionality of the articulation.
- Litwic A, Edwards MH, Dennison EM, Cooper C (2013) Epidemiology and burden of osteoarthritis. Br Med Bull 105: 185-199. [Crossref]
- Reginster JY, Bruyere O, Fraikin G, Henrotin Y (2005) Current concepts in the therapeutic management of osteoarthritis with glucosamine. Bull Hosp Jt Dis 63: 31-36. [Crossref]
- Largo R, Alvarez-Soria MA, Diez-Ortego I, Calvo E, Sanchez-Pernaute O, et al. (2006) Glucosamine inhibits IL-1beta-induced NFkappaB activation in human osteoarthritic chondrocytes. Osteoarthr Cartil 11: 290-298.
- Alvarez-Soria MA, Largo R, Calvo E, Herrero-Beaumont G (2005) Differential anticatabolic profile of glucosamine sulfate versus other anti-osteoarthritic drugs on human osteoarthritic chondrocytes and synovial fibroblasts in culture. Osteoarthr Cartil 13: S153.
- Uitterlinden EJ, Jahr H, Koevoet JL, Jenniskens YM, Bierma-Zeinstra SM, et al. (2006) Glucosamine decreases expression of anabolic and catabolic genes in human osteoarthritic cartilage explants. Osteoarthr Cartil 14: 250-257.
- Taniguchi S, Ryu J, Seki M, Sumino T, Tokuhashi Y, et al. (2012) Long-term oral administration of glucosamine or chondroitin sulfate reduces destruction of cartilage and up-regulation of MMP-3 mRNA in a model of spontaneous osteoarthritis in Hartley guinea pigs. J Orthop Res 30: 673-678. [Crossref]
- Imagawa K, Andres MC, Hashimoto K, Pitt D, Itoi E, et al. (2011) The epigenetic effect of glucosamine and a nuclear factor-kappa B (NF-kB) inhibitor on primary human chondrocytes-implications for osteoarthritis. Biochem Biophys Res Commun 405: 362–367.
- Altman RD, Abramson S, Bruyere O, Clegg D, Herrero-Beaumont G, et al. (2006) Commentary: osteoarthritis of the knee and glucosamine. Osteoarthr Cartil 14: 963–966.
- Reginster JY, Bruyere O, Neuprez A (2007) Current role of glucosamine in the treatment of osteoarthritis. Rheumatology (Oxford) 46: 731-735. [Crossref]
- Reichelt A, Förster KK, Fischer M, Rovati LC, Setnikar I (1994) Efficacy and safety of intramuscular glucosamine sulfate in osteoarthritis of the knee. A randomised, placebo-controlled, double-blind study. Arzneimittelforschung 44: 75-80. [Crossref]
- Setnikar I, Palumbo R, Canali S, Zanolo G (1993) Pharmacokinetics of glucosamine in man. Arzneimittelforschung 43: 1109-1113. [Crossref]
- Leffler CT, Philippi AF, Leffler SG, Mosure JC, Kim PD (1999) Glucosamine, chondroitin, and manganese ascorbate for degenerative joint disease of the knee or low back: a randomized, double-blind, placebo-controlled pilot study. Mil Med 164: 85-91. [Crossref]
- Rovati L (1997) Clinical development of glucosamine sulfate as selective drug in osteoarthritis. Rheumatol Eur pp: 26:70.
- Houpt JB, McMillan R, Wein C, Paget-Dellio SD (1999) Effect of glucosamine hydrochloride in the treatment of pain of osteoarthritis of the knee. J Rheumatol 26: 2423-2430. [Crossref]
- Rovati L (1993) Clinical efficacy of glucosamine sulfate in osteoarthritis of the spine. Rev Esp Reumatol 20(S1): 325.
- Müller-Fassbender H, Bach GL, Haase W, Rovati LC, Setnikar I (1994) Glucosamine sulfate compared to ibuprofen in osteoarthritis of the knee. Osteoarthritis Cartilage 2: 61-69. [Crossref]
- Qiu GX, Gao SN, Giacovelli G, Rovati L, Setnikar I (1998) Efficacy and safety of glucosamine sulfate versus ibuprofen in patients with knee osteoarthritis. Arzneimittelforschung 48: 469-474. [Crossref]
- Rovati L (1997) The clinical profile of glucosamine sulfate as a selective symptom modifying drug in osteoarhtritis: current data and perspective. Osteoarthr Cartil 5(SA): 72.
- Reginster JY, Neuprez A, Lecart MP, Sarlet N, Bruyere O (2012) Role of glucosamine in the treatment for osteoarthritis. Rheumatol Int 32: 2959-2967. [Crossref]
- Clegg DO, Reda DJ, Harris CL, Klein MA, O'Dell JR, et al. (2006) Glucosamine, chondroitin sulfate, and the two in combination for painful knee osteoarthritis. N Engl J Med 354: 795-808. [Crossref]
- Peregoy J, Wilder FV (2011) The effects of vitamin C supplementation on incident and progressive knee osteoarthritis: a longitudinal study. Public Health Nutr 14: 709-715. [Crossref]
- Malek Mahdavi A, Mahdavi R, Kolahi S, Zemestani M, Vatankhah AM. L-Carnitine supplementation improved clinical status without changing oxidative stress and lipid profile in women with knee osteoarthritis. Nutr Res 35: 707-715.
- Oben J, Enonchong E, Kothari S, Chambliss W, Garrison R, et al. (2009) Phellodendron and Citrus extracts benefit joint health in osteoarthritis patients: a pilot, double-blind, placebo-controlled study. Nutr J 8:38.
- Witt CM, Michalsen A, Roll S, Morandi A, Gupta S, et al. (2013) Comparative effectiveness of a complex Ayurvedic treatment and conventional standard care in osteoarthritis of the knee. Study protocol for a randomized controlled trial. Trials 14: 149.
- Ezaki J, Hashimoto M, Hosokawa Y, Ishimi Y (2013) Assessment of safety and efficacy of methylsulfonylmethane on bone and knee joints in osteoarthritis animal model. J Bone Miner Metab 31: 16-25. [Crossref]
- McAlindon TE, Nuite M, Krishnan N, Ruthazer R, Price LL, et al. (2011) Change in knee osteoarthritis cartilage detected by delayed gadolinium enhanced magnetic resonance imaging following treatment with collagen hydrolysate: a pilot randomized controlled trial. Osteoarthritis Cartilage 19: 399-405. [Crossref]
- Bannuru RR, Osani M, Vaysbrot EE, McAlindon TE (2016) Comparative safety profile of hyaluronic acid products for knee osteoarthritis: a systematic review and network meta-analysis. Osteoarthritis Cartilage S1063-4584.
- Walrand S, Chiotelli E, Noirt F, Mwewa S, Lassel T (2008) Consumption of a functional fermented milk containing collagen hydrolysate improves the concentration of collagen-specific amino acids in plasma. J Agric Food Chem 56: 7790-7795.
- Bello AE, Oesser S (2006) Collagen hydrolysate for the treatment of osteoarthritis and other joint disorders: a review of the literature. Curr Med Res Opin 22: 2221-2232. [Crossref]
- Oesser S, Seifert J (2003) Stimulation of type II collagen biosynthesis and secretion in bovine chondrocytes cultured with degraded collagen. Cell Tissue Res 311: 393-399. [Crossref]
- Schunck M, Schulze CH, Oesser S (2006) Disparate efficacy of collagen hydrolysate and glucosamine on the extracellular matrix metabolism of articular chondrocytes. OA and Cartilage 14: S114.
- Oesser S, Proksch E, Schunck M (2008) prophylactic treatment with a special collagen hydrosylate decreases cartilage tissue degeneration in the knee joints. OA and Cartilage 16: S45.
- Nakatani S, Mano H, Sampei C, Shimizu J, Wada M (2009) Chondroprotective effect of the bioactive peptide prolyl-hydroxyproline in mouse articular cartilage in vitro and in vivo. Osteoarthritis Cartilage 17: 1620–1627.
- Clark KL, Sebastianelli W, Flechsenhar KR, Aukermann DF, Meza F, et al. (2008) 24-Week study on the use of collagen hydrolysate as a dietary supplement in athletes with activity-related joint pain. Curr Med Res Opin 24: 1485-1496. [Crossref]
- Zuckley L, Angelopoulou K, Carpenter MSS, Meredith BA, et al. (2004) Collagen hydrosylate improves joint function in adults with mild symptoms of osteoarthritis of the knee. Medicine & Science in Sports & Exercise 36: S153–S154.
- Moskowitz RW (2000) Role of collagen hydrolysate in bone and joint disease. Semin Arthritis Rheum 30: 87-99. [Crossref]
- Benito-Ruiz P, Camacho-Zambrano MM, Carrillo-Arcentales JN, Mestanza-Peralta MA, Vallejo-Flores CA, et al. (2009) A randomized controlled trial on the efficacy and safety of a food ingredient, collagen hydrolysate, for improving joint comfort. Int J Food Sci Nutr 60: 99–113
- Fitton JH (2011) Therapies from fucoidan; multifunctional marine polymers. Mar Drugs 9: 1731-1760. [Crossref]
- Park SB, Chun KR, Kim JK, Suk K, Jung YM, et al. (2010) The differential effect of high and low molecular weight fucoidans on the severity of collagen-induced arthritis in mice. Phytother Res 24: 1384-1391. [Crossref]
- Myers SP, O Connor J, Fitton JH, Brooks L, Rolfe M, Connellan P, et al. (2010) A combined phase I and II open label study on the effects of a seaweed extract nutrient complex on osteoarthritis. Biologics 4: 33-44.
- Kiple KF, Ornelas KC (2000) Important Vegetable Supplements. In: Beck SV (Ed) The Cambridge World History of Food. Cambridge University Press, Cambridge, UK 1: 231–249.
- Berteau O, Mulloy B (2003) Sulfated fucans fresh perspectives: Structures, functions, and biological properties of sulfated fucans and an overview of enzymes active toward this class of polysaccharide. Glycobiolog 13: 29–40.
- Myers SP, O’Connor J, Fitton JH, Brooks L, Rolfe M, et al. (2011) A combined Phase I and II open-label study on the immunomodulatory effects of seaweed extract nutrient complex. Biologics 5: 45–60.
- Cunha TM, Verri WA Jr, Schivo IR, Napimoga MH, Parada CA, et al. (2008) Crucial role of neutrophils in the development of mechanical inflammatory hypernociception. J Leukoc Biol 83: 824-832. [Crossref]
- Lequesne MG (1997) The algofunctional indices for hip and knee osteoarthritis. J Rheumatol 24: 779-781. [Crossref]
- Raatz SK, Redmon JB, Wimmergren N, Donadio JV, Bibus DM (2009) Enhanced absorption of n-3 fatty acids from emulsified compared with encapsulated fish oil. J Am Diet Assoc 109: 1076-1081. [Crossref]
- Felson DT, Zhang Y (1998) An update on the epidemiology of knee and hip osteoarthritis with a view to prevention. Arthritis Rheum 41: 1343-1355. [Crossref]
- Matheson AJ, Perry CM (2003) Glucosamine: a review of its use in the management of osteoarthritis. Drugs Aging 20: 1041-1060. [Crossref]
- Uebelhart D, Thonar EJ, Delmas PD, Chantraine A, Vignon E (1998) Effects of oral chondroitin sulfate on the progression of knee osteoarthritis: a pilot study. Osteoarthritis Cartilage 6 (suppl A): 39-46.




